More Information
Submitted: July 05, 2021 | Approved: November 08, 2021 | Published: November 09, 2021
How to cite this article: Gadzama N, Ahmed I, Khursheed S, Rizwan F, AL-Assaf N, et al. The role of urine metabolomics among newborn infants with hypoxic ishaemic encephalopathy: a literature review. J Adv Pediatr Child Health. 2021; 4: 109-113.
DOI: 10.29328/journal.japch.1001042
Copyright License: © 2021 Gadzama N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Urine metabolomics; Biomarker; Perinatal asphyxia; Hypoxic ischaemic encephalopathy
The role of urine metabolomics among newborn infants with hypoxic ishaemic encephalopathy: a literature review
Nese Gadzama1,2*, Irfan Ahmed2, Sundus Khursheed3, Fizza Rizwan4, Niazy AL-Assaf1 and Rizwan Khan1
1Department of Neonatology, University Maternity Hospital Limerick, Limerick, Republic of Ireland
2Department of Paediatrics, University Hospital Limerick, Limerick, Republic of Ireland
3Department of Paediatrics, University Hospital Kerry, Tralee, Republic of Ireland
4Second Year Medical Student, Medical University of Sofia, Bulgaria
*Address for Correspondence: Dr. Nese Gadzama, Department of Paediatrics, University Hospital Limerick, Dooradoyle, Co. Limerick, Republic of Ireland, Email: [email protected]; [email protected]; [email protected]
Background: Perinatal asphyxia (PA) which may result in hypoxic ischaemic encephalopathy (HIE) affects four million neonates worldwide and accounts for the death of one million of affected babies. The science of metabolomics has become an area of growing interest in neonatal research, with a potential role in identifying useful biomarkers that can accurately predict injury severity in perinatal asphyxia and HIE.
The aim of this review is to look at the evidence of the usefulness of urine metabolomics in predicting outcome in PA/HIE.
Methods: The key words used in the advanced search ‘urine metabolomics’ AND ‘perinatal asphyxia’ OR ‘hypoxic ischaemic encephalopathy’, yielded 13 articles.
Results: Of the selected thirteen studies, 38% (n = 5) were human studies, 31% (n= 4) were animal studies and 31% (n = 4) were review articles. The studies confirmed the involvement of known pathways in the development of PA/HIE, primarily the Krebs cycle evidenced by accumulation of TCA cycle intermediates (citrate, α-ketoglutarate, succinate) and anaerobic pathways indicated by increased lactate. Other pathways involved include amino acid and carbohydrate pathways.
Conclusion: Metabolomic studies so far are promising in highlighting potential biomarker profiles in PA/HIE. Further research is necessary to further clarify the role of identified metabolites in predicting outcome and prognosis in neonates affected by PA/HIE.
Perinatal asphyxia (PA) is an insult to the fetus immediately before, during or after birth which occurs as a result of oxygen deprivation resulting in hypoperfusion [1]. Every year, it affects four million neonates worldwide causing the death of one million of these babies [1]. Some of the affected neonates go on to develop hypoxic ischaemic encephalopathy (HIE) which results in permanent neurological and multi-organ impairment in varying severity [1].
HIE is a heterogeneous, multi-faceted disease entity which passes through different stages after the hypoxic insult has occurred [15]. The metabolic response to hypoxia remains unclear [2] and as such, we cannot rely on the use of a single or small number of biomarkers in isolation to provide high selectivity and sensitivity for predicting clinical outcomes [2,15]. Given its multifactor dependency, the timing, severity and outcome of this disease is difficult to predict [1]. The search for useful biomarkers to accurately predict injury severity in perinatal asphyxia and hypoxic ischaemic encephalopathy has become an area of growing interest in neonatal research [2]. Despite the potential of many promising markers, few studies have studied their validity or addressed their application in clinical practice [3].
Metabolomics, the newest of the ‘omics’ science studies the complete set of metabolites in a given biological sample by identifying, quantifying and characterizing hundreds and even thousands of low molecular weight metabolites simu-ltaneously, in a systemic manner [4]. It is capable of producing a snapshot of the metabolome, the entire complement of low molecular weight metabolites produced by an organism, a mirror that reflects the physiological, evolutionary and pathological state of a biological system [5].
The metabolomic approach combines high throughput analysis (multivariate analysis of the data matrices produced) with theory, bioinformatics and computational statistics [2,4]. Biological samples such as blood, urine, plasma and cerebrospinal fluid are the most commonly used and the choice of sample depends on the objective of the study [4]. Urine as a biological sample is particularly useful for metabolomic analysis, as it is possible to analyze a large number of compounds which reflect different metabolic processes [4]. Urine is considered as a ‘window into the organism’ given its characteristic biochemical composition which is the result of a combination of factors such as genotype, physiological state, health status, nutrition, drugs and the environment [4]. The ability of metabolomics to measure rapid alterations in metabolism has potential to clarify disease mechanisms in perinatal asphyxia by the qualitative or quantitative description of the activation and interaction of metabolic pathways, ultimately portraying an infant’s phenotype [4,16].
In this review, we aim to look at the evidence on the usefulness of urine metabololites in predicting outcome in PA/HIE.
PubMed, Science Direct and Embase were searched using the key words (urine metabolomics) AND (perinatal asphyxia) OR (hypoxic ischaemic encephalopathy).
Inclusion criteria were: studies performed between 2000 – 2020 and articles in English.
Exclusion criteria were: studies performed before 2000, abstract only, articles not in English.
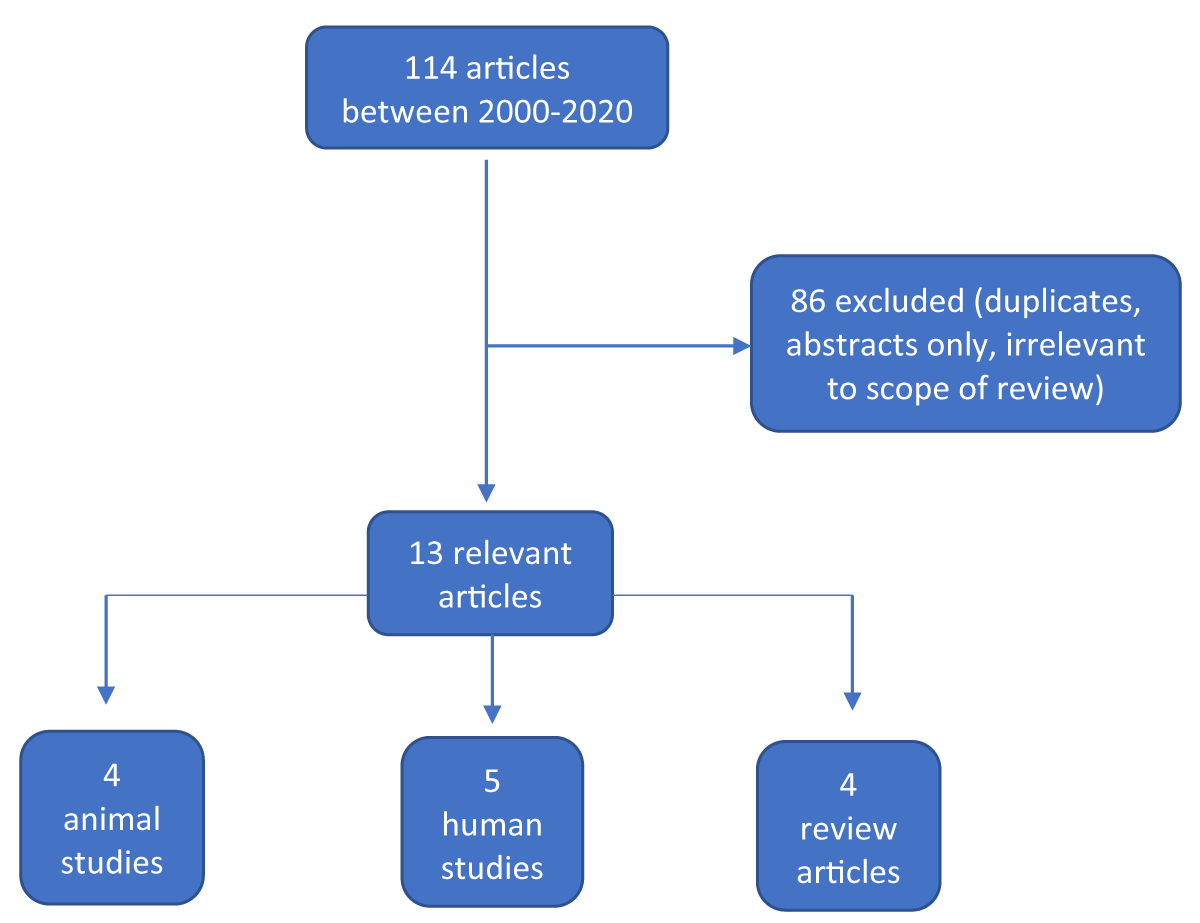
The search yielded 114 articles, of which 13 were specifically related to urine metabolomics in perinatal asphyxia and or HIE. Duplicate articles were removed. Of the 13 articles, 4 were reviews (31%), 4 were animal studies (31%) and 5 were human studies (38%) (Figure 1).
Figure 1: Summary of results and selection process.
Analytical methods used in the studies included mass spectrometry (MS) and nuclear magnetic resonance spectro-scopy (H-NMR), with varied metabolic profiling and results, details of which are summarized in Table 1 and Table 2 for animal and human studies respectively.
| Table 1: Summary of Urine Metabolomic studies in Animal models with Perinatal Asphyxia/HIE. | |||||
| Author/Year | Study Type (Experimental Model) | Aim | Analysis/Sample | Results | Specific Observations |
| Atzori, et al. [6] | Randomized control (Newborn Piglets) |
Characterize the metabolic profiles of newborn hypoxic-reoxygenated piglets. | H-NMR - urine | Metabolic alterations were observed in the different oxygen treated groups. The most important discriminating metabolites were - urea, creatinine, malonate, methylguanidine, hydroxyisobutyric acid. |
Metabolic profiles of piglets in asystole differed from those not in asystole – suggesting that a different metabolic phenotype could be associated with the response to oxygen therapy. |
| Skappak, et al. [7] | Randomized control (Newborn Piglets) |
Identify hypoxia and establish a metabolomic profile of neonatal hypoxia-reoxygenation. | NMR - urine | 13 urinary metabolites differentiated hypoxic from non-hypoxic piglets. These were – alanine, asparagine, betaine, citrate, creatine, fumarate, Hippurate, lactate, 1-methylnicotinamide, N-acetylglycine, N-carbamoyl-β-alanine, 2-oxoglutarate, valine. The PLS-DA model correctly diagnosed the blinded hypoxic samples with 90% accuracy. Both groups had similar physiologic measurements after the period of recovery, but with differing urine metabolite profiles. |
No single metabolite could diagnose all animals correctly. For example, lactate was elevated in some non-hypoxic animals – suggesting that to have accuracy at diagnosis a combination of variables will be needed. |
| Fanos, et al. [8] | Case-control study (Newborn piglets) |
Investigate whether metabolomic profiles differed based on oxygen concentration received at resuscitation. | H-NMR - urine | 21% FiO2 is the most “physiological” and appropriate concentration to be used for resuscitation. | The metabolomic approach used clearly identified four different metabolic signatures related to the different oxygen concentration treatments given to the animals. |
| Sachse, et al. [9] | Randomized study (Newborn piglets) | Identify biomarkers for subject characterization, intervention effects and possibly prognosis. | NMR – urine and plasma | Plasma and urine showed severe metabolic alterations consistent with hypoxia and acidosis at 2 and 4 hours after ROSC. Baseline plasma hypoxanthine and lipoprotein concentrations were inversely correlated to the duration of hypoxia sustained before asystole. There was no evidence for a differential metabolic response to the different resuscitation protocols or in terms of survival. |
The study demonstrated the severe metabolic alterations in plasma and urine caused by asphyxia-induced cardiac arrest and CPR. The metabolic profiles could potentially be useful in predicting outcome after hypoxic insults in newborn pigs and thereby infants and for future guidance for resuscitation strategy. |
| NMR: Nuclear Magnetic Resonance (spectroscopy): PLS-DA: Partial Least Squares-Discriminant Analysis; ROSC: Return of Spontaneous Circulation; CPR: Cardiopulmonary Resuscitation. | |||||
| Table 2: Summary of Urinary Metabolomic Human studies with Perinatal Asphyxia/HIE. | |||||
| Author/Year | Study type (Experimental model) | Aim | Analysis/Sample | Results | Specific Observations |
| Chu, et al. [10] | Prospective case-control study (Asphyxiated neonates) |
Studied urine metabolomic profiles of neonates with severe birth asphyxia and subsequent neurodevelopmental handicap. | Mass spectrometry – urine |
Urinary organic acids associated with good outcome: ethylmalonate, 3-OH-3-methylglutarate, 2-OH-glutarate and 2-oxo-glutarate. Urinary organic acids associated with poor outcome: glutarate, methylmalonate, 3-OH-butyrate and orate. |
Metabolomic discriminators between good and poor neonatal outcome were established. |
| Longini, et al. [11] | Prospective case-control study (Pre-term asphyxiated neonates) |
Evaluate the effects of asphyxia on newborn urine metabolites and highlight differences in the metabolic profile of healthy (control) compared with asphyxiated newborns. | H-NMR spectroscopy - urine | Metabolites characterizing the asphyxiated group were: lactate, glucose, TMAO, threonine and 3-hydroxyisovalerate. 24-48 hours after resuscitation, preterm asphyxiated neonates showed a recovery pattern that could still be differentiated from the controls. |
Asphyxiated neonates were distinguished from the controls by the difference in their metabolic profiles which was obtained through H-NMR spectroscopy. |
| Noto, et al. [12] | Longitudinal experimental study (Newborns with HIE undergoing hypothermia) | Identify the urine metabolome of newborns with perinatal asphyxia and to follow its changes over time. | GC-MS - urine | The urine metabolic profile of the neonates who died on day 7 of life were comparable to each other and significantly different from that of survivors. | Assumption that taurine may be considered a candidate biomarker for assessing and monitoring cellular injury and death during a hypoxic-anoxic insult. |
| Sarafidis, et al. [13] | Prospective case-control study (Newborns with HIE) | Identify changes in the urine metabolome of neonates with HIE compared to healthy controls. | LC-MS/MS - urine | A clear separation between the HIE and control groups was identified, with pyruvic acid, amino acids, acylcarnitines, inositol, kynurenine, hippuric acid and vitamins as the discriminant metabolites. | A specific metabolic profile in neonates with HIE was identified through targeted metabolomic analysis. |
| Locci, et al. 2018 [14] | Prospective case-control study (Newborns with HIE undergoing hypothermia) | Identify metabolites associated with HIE and to follow their modifications over time. | H-NMR spectroscopy - urine | There were significant differences in the urine metabolome of HIE and healthy newborns. The changes in the urine metabolome of the HIE group reflected either the effects of the TH or the physiological growth of the newborns. |
Only the non-surviving HIE newborns presented a ‘pathological’ lactate at the semi-quantitative level, while the corresponding values in the surviving HIE babies was similar to the healthy controls. |
| OH: Hydroxy; H-NMR: Nuclear Magnetic Resonance (spectroscopy); TMAO: Trimethylamine N-oxide, HIE: Hypoxic Ischaemic Encephalopathy; GC-MS: Gas Chromatography-Mass Spectrometry; LC-MS/MS: Liquid Chromatography with Tandem Mass Spectrometry. | |||||
The reviewed studies confirmed the involvement of known pathways in the development of PA/HIE, mainly the Krebs cycle indicated by accumulation of TCA cycle intermediates (citrate, α-ketoglutarate, succinate) [8,12,14] and anaerobic pathways indicated by increased lactate [7-9,11-12,14]. Summary of the studies and their results are found in Tables 1 and 2 for animal and human studies respectively.
During the asphyxia event, activation of the anaerobic glycolysis pathway leads to increased production of lactate, the accumulation of which induces acidosis, one of the parameters used to determine the need for therapeutic hypothermia (TH) [14]. Lactate is an alternative source of energy for neurons when there is reduced availability of glucose [14] and it has been shown to be a predictor of poor outcome in human studies [12,14]. Locci, et al. [14] were able to show that urinary lactate in the babies who died was markedly elevated and remained so in one case, while the surviving HIE newborns showed a similar urinary lactate level to the babies in the control group (full term healthy newborns) [14], this was also demonstrated by Skappak, et al. [7]. In a urine and plasma metabolomic study of asphyxiated newborn pigs, Sachse, et al. [9], found that tracing the lactate concentrations over time revealed a lag between plasma and urine: while plasma lactate levels increased and then started to decrease over the studied time intervals, urinary lactate levels stayed high or even increased slightly more.
A similar lag pattern was also observed for the concentration of other metabolites, in particular the branch chain amino acids (BCAAs) [9]. This is in contrast to the findings of Sarafidis, et al. [13] in a urine metabolomic profile in neonates with HIE, where BCAAs (valine, leucine, isoleucine) as well as aromatic (phenylalanine, tyrosine, tryptophan) and neutral (threonine) amino acids decreased in the urine of neonates with HIE on the first day of life compared to controls [13]. BCAAs and aromatic amino acids are biochemical precursors of important neurotransmitters including dopamine, serotonin and melatonin [13]. Chronic hypoxia/ischaemia reduces the activity of the enzymes involved in the synthesis of these neurotransmitters from their precursor amino acids, causing neuronal deficiency [12]. Threonine is an essential amino acid in humans which works as an immunostimulant, promoting the growth of the thymus gland and probably promote cell immune defense function [11].
Citric acid and other TCA cycle intermediates (α-ketoglutarate, succinate,) were found to be increasing over time in the urine of babies with HIE [12,14]. Sachse, et al. [9] also found a similar increase in plasma and to a lesser extent urine succinate and fumarate levels in a metabolomic study of asphyxiated newborn piglets. The TCA cycle is the central metabolic hub of the cell, being the gateway to the aerobic metabolism of any molecule that can be transformed into an acetyl group or dicarboxylic acid [12]. The increase of these metabolites over time can be correlated with the progressive re-activation of oxygen-dependent ATP production pathways, after the hypoxic-ischaemic event [12,14]. Noto, et al. [12] found high levels of 2-ketoglutaric acid in the urine of babies who survived both at birth and at 72 hours, strongly supporting the assumption that in these babies the aerobic pathways have been re-activated and its concomitant absence in dead babies [12].
Acetate, a precursor of acetyl-co-enzyme A, which has a central role in the metabolism of carbohydrates and fats by entering the TCA cycle [17] was shown to be decreased in babies with HIE [11,14]. Glucose was found to be increased in the urine of asphyxiated neonates [8,11], while there was a decrease in fructose over time alongside the simultaneous increase of lactose and galactose [12]. Fructose is metabolized in the liver, primarily to glycogen; its accumulation at birth and subsequent decrease are consistent with the recovery of liver function over time [12]. Lactose is present in milk and it is the primary carbohydrate source for mammals. It is a disaccharide of D-glucose and D-galactose, constituting 40% of the energy consumed by humans in the nursing period and its progressive increase is as a result of feeding in survivors [12].
Finally, taurine, an intracellular metabolite may be considered as a biomarker of cellular injury and death, as alterations in plasma membrane permeability and cytolysis give rise to the accumulation of extracellular taurine [12]. Noto, et al. [12] found a decrease in urine Tuarine levels over time in babies who survived and increased over time in the babies who died.
In conclusion, animal studies have helped in elucidating the metabolites involved in PA/HIE, however, it is difficult to assess short- and long-term outcomes given their nature and design. Human metabolomic studies till date have shown the promising field of developing a biomarker profile in PA/HIE to assess babies but these have had little to no clinical application given the size, their design and power of the studies. Given the relative ease to collect urine samples and the requirement to take biological samples over time, urine metabolomics will play a vital role in the development of biomarkers of PA/HIE. It is important to note that the concentration of urine metabolites depends not only on the plasma concentrations of the metabolites, but also on the renal handling of individual metabolites and sampling frequency [9]. With this in mind, we recommend a well-designed multi-center randomized controlled trial which will combine urine and plasma metabolomics in order to further clarify the role of identified metabolites in predicting outcome and prognosis in neonates affected by PA/HIE.
I would like to thank Dr. Irfan, Dr. Khan and all the team at the University Maternity Hospital Limerick, for their guidance in the writing of this review.
Declarations
Consent for publication: The authors declare they have all given consent for the publication of this manuscript.
- Fattuoni C, Palmas F, Noto A, Fanos V, Barberini L. Perinatal asphyxia: a review from a metabolomics perspective. Molecules (Basel, Switzerland). 2015; 20: 7000–7016. PubMed: https://pubmed.ncbi.nlm.nih.gov/25898414/
- Denihan NM, Boylan GB, Murray DM. Metabolomic profiling in perinatal asphyxia: a promising new field. BioMed Res Int. 2015: 254076. PubMed: https://pubmed.ncbi.nlm.nih.gov/25802843/
- Ramaswamy V, Horton J, Vandermeer B, Buscemi N, Miller S, et al. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatric Neurol. 40: 215–226. PubMed: https://pubmed.ncbi.nlm.nih.gov/19218035/
- Fanos V, Van den Anker J, Noto A, Mussap M, Atzori L. Metabolomics in neonatology: fact or fiction? Semin Fetal Neonatal Med. 2013; 18: 3–12. PubMed: https://pubmed.ncbi.nlm.nih.gov/23195852/
- Baker M. Metabolomics: from small molecules to big ideas. Nat Methods. 2011; 8: 117–121.
- Atzori L, Xanthos T, Barberini L, Antonucci R, Murgia F, et al. A metabolomic approach in an experimental model of hypoxia-reoxygenation in newborn piglets: urine predicts outcome. J Matern Fetal Neonatal Med. 2010; 23 Suppl 3: 134–137. PubMed: https://pubmed.ncbi.nlm.nih.gov/20873980/
- Skappak C, Regush S, Cheung PY, Adamko DJ. Identifying hypoxia in a newborn piglet model using urinary NMR metabolomic profiling. PloS ONE. 8: e65035. PubMed: https://pubmed.ncbi.nlm.nih.gov/23741447/
- Fanos V, Noto A, Xanthos T, Lussu M, Murgia F, et al. Metabolomics network characterization of resuscitation after normocapnic hypoxia in a newborn piglet model supports the hypothesis that room air is better. BioMed Res Int. 2014; 731620. PubMed: https://pubmed.ncbi.nlm.nih.gov/24696864/
- Sachse D, Solevåg AL, Berg JP, Nakstad B. The Role of Plasma and Urine Metabolomics in Identifying New Biomarkers in Severe Newborn Asphyxia: A Study of Asphyxiated Newborn Pigs following Cardiopulmonary Resuscitation. PloS ONE. 2016; 11: e0161123. PubMed: https://pubmed.ncbi.nlm.nih.gov/27529347/
- Chu CY, Xiao X, Zhou XG, Lau TK, Rogers MS, et al. Metabolomic and bioinformatic analyses in asphyxiated neonates. Clinical Biochem. 2006; 39: 203–209. PubMed: https://pubmed.ncbi.nlm.nih.gov/16460720/
- Longini M, Giglio S, Perrone S, Vivi A, Tassini M, et al. Proton nuclear magnetic resonance spectroscopy of urine samples in preterm asphyctic newborn: a metabolomic approach. Clin Chim Acta. 2015; 444: 250–256. PubMed: https://pubmed.ncbi.nlm.nih.gov/25727514/
- Noto A, Pomero G, Mussap M, Barberini L, Fattuoni C, et al. Urinary gas chromatography mass spectrometry metabolomics in asphyxiated newborns undergoing hypothermia: from the birth to the first month of life. Ann Transl Med. 2016; 4: 417. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5124630/
- Sarafidis K, Efstathiou N, Begou O, Soubasi V, Agakidou E, et al. Urine metabolomic profile in neonates with hypoxic-ischemic encephalopa-thy. Hippokratia. 2017; 21: 80–84. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6239088/
- Locci E, Noto A, Puddu M, Pomero G, Demontis R, et al. A longitudinal 1H-NMR metabolomics analysis of urine from newborns with hypoxic-ischemic encephalopathy undergoing hypothermia therapy. PloS ONE. 2018; 13: e0194267. PubMed: https://pubmed.ncbi.nlm.nih.gov/29668681/
- Sánchez-Illana Á, Piñeiro-Ramos JD, Kuligowski J. Small molecule biomarkers for neonatal hypoxic ischemic encephalopathy. Semin Fetal Neonatal Med. 2020; 25: 101084. PubMed: https://pubmed.ncbi.nlm.nih.gov/31983670/
- Reinke SN, Broadhurst DI. Moving metabolomics from a data-driven science to an integrative systems science. Gen Med. 2012; 4: 85.
- Efstathiou N, Theodoridis G, Sarafidis K. Understanding neonatal hypoxic-ischemic encephalopathy with metabolomics. Hippokratia. 2017; 21: 115–123. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6248003/