More Information
Submitted: April 15, 2022 | Approved: July 01, 2022 | Published: July 04, 2022
How to cite this article: Krizam G, Almulla A, Lootah A, Alkatib T, Altatari H. Can infants develop meningitis in the absence of bacteremia in the first ninety days of life? A retrospective chart review. J Adv Pediatr Child Health. 2022; 5: 022-025.
DOI: 10.29328/journal.japch.1001047
Copyright License: © 2022 Krizam G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Can infants develop meningitis in the absence of bacteremia in the first ninety days of life? A retrospective chart review
Krizam G* , Almulla A, Lootah A, Alkatib T and Altatari H
, Almulla A, Lootah A, Alkatib T and Altatari H
Sheikh Khalifa Medical City Ajman, United Arab Emirates
*Address for Correspondence: Ghada Krizam, Sheikh Khalifa Medical City Ajman, United Arab Emirates, Email: [email protected]
The overall incidence of meningitis in infants 0-90 days is low; however, it remains a serious cause of morbidity and mortality among affected patients. It is standard of care to perform lumbar punctures as part of the work-up of fever in the first four weeks of life and sick-looking babies up to the age of 90 days. This particular procedure is often refused by parents, and physicians are left to predict the possibility of meningitis based on blood culture results.
The aim of this study is to determine whether it would be safe to rule out meningitis based on a negative blood culture in this age group.
Bacterial meningitis is more common in the first month than at any other time of life [1]. It is associated with high mortality and long-term complications among survivors in the neonatal period [2]. Since signs and symptoms of meningitis are often subtle and non-specific in infants, clinicians often use laboratory studies to evaluate for early-onset and late-onset sepsis including a complete blood count (CBC) and differential, blood and cerebrospinal fluid (CSF) cultures, and measurement of levels of C-reactive protein (CRP) and possibly other infection markers. However, recognition of neonatal meningitis continues to be a challenge and it has been reported that neonatal meningitis can occur in the absence of bacteremia; and that no single cerebrospinal fluid (CSF) value can exclude its presence [3]. The present study aims to find out the concordance between blood, and CSF cultures as well as other inflammatory markers and urine cultures in infants between 0-90 days of life treated in Tawam Hospital, between the years 2008-2017. Tawam Hospital is one of the major tertiary hospitals in the United Arab Emirates with about 154 beds dedicated to different pediatric units.
Study design
This is a retrospective chart review for all infants aged zero to 90 days who underwent a complete sepsis workup including a lumbar puncture between the years 2008 and 2017. The following information was collected from patient charts: gestational age, age at which lumber puncture was performed, mode of delivery, maternal risk factors (GBS status & prolonged rupture of membranes), and maternal parity number. Peripheral White Blood Cell (WBC) count and C-Reactive Protein (CRP) level at the time of admission were also collected along with blood, CSF, and urine culture growths and the time to positivity of any growth. The results of enterovirus Polymerase Chain reaction (PCR) from the CSF samples were also collected, along with the results of the CSF analysis which included WBC count, Red Blood Cells (RBC) count, Protein level & Glucose level.
Data interpretation
As the results of the study are descriptive, no specific data analysis software was used. Data was entered in an excel sheet. Then only patients with a positive CSF culture were further studied and analyzed.
Patient confidentiality
Only the investigators of the study had access to patients’ files. During data collection, each patient was identified by their specific hospital number only; no patient names were used. Once the data collection phase was completed, all the cases were merged into a single document which was only available to the investigators of the study.
Outcomes
The primary outcome of the study was to examine the possibility of having meningitis in the absence of a positive blood culture during the first three months of life.
The secondary outcomes of the study included studying the correlation between the patient’s age and the presence of meningitis, and whether there are any maternal factors that would increase the risk of such an infection. We also examined the levels of CRP and WBC counts in correlation to meningitis and bacteremia. Finally, CSF analyses were looked at to see how likely they were to predict CSF culture positivity.
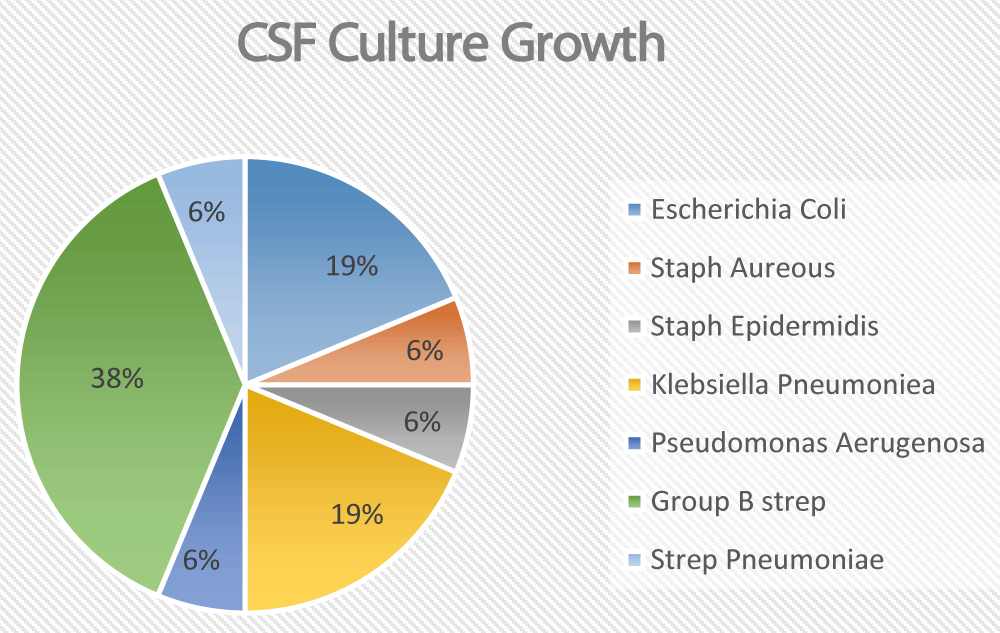
The total number of reviewed charts was 607. Seven patients were excluded due to a lack of information regarding blood and CSF culture results. Of the remaining 600 patients, 11 patients had a positive CSF PCR for enterovirus. 41 patients had CSF samples with positive bacterial cultures. However, 25 of them were deemed contaminants and were excluded. That left us with 16 CSF samples with real bacterial growth belonging to 16 different patients (Figure 1). Those 16 patients were further analyzed in detail.
Figure 1: Break down of the CSF Culture growths
Five out of the 16 bacterial CSF growths (31% of all positive cases) had negative blood cultures.
None of the patients with positive CSF cultures had a positive urine culture. The main culprit pathogen was Group B streptococcus. Other common pathogens included E. coli and Klebsiella Pneumonia. One patient had Pseudomonas aeruginosa. Only one patient grew Streptococcus Pneumonia and one patient with ventriculoperitoneal shunt grew staphylococcus epidermidis (Table 1).
| Table 1: Analysis of bacterial growths from CSF cultures and their main characteristics | |||
| Bacteria isolated | Percentage of patients who had a corresponding negative blood culture |
Age of affected patients | Identifiable risk factors in the affected patients |
| Escherichia Coli | 0% | 0-2 weeks old | No risk factors identified |
| Streptococcus agalactiae (Group B Strep) |
33% | 0-6 weeks old. More than 80% of patients under the age of 3 weeks | 50% of patients had a confirmed Positive GBS status in the mother |
| Klebsiella Pneumoniae | 66% | 3-19 weeks old. 66% of patients were 3 weeks old | No risk factors identified |
| Other organisms | 25% | 1-13 weeks old | No risk factors identified |
7 out of the 16 patients were preterm, while the remaining 9 were born at 37 weeks or more (term infants).
White blood cell count on the admission of all patients with true CSF bacterial growth was either normal or low. Only 2 patients had significant leukocytosis, but they were both extremely preterm and were either in very critical condition or on steroids. Patients who were found to have enterovirus meningitis all had normal WBC counts.
Unfortunately, CRP levels were not helpful in distinguishing those patients who ended up with a positive culture be it blood CSF or urine. Although most of the time patients with positive blood or CSF cultures had elevated CRP, there were 24 patients who had positive blood or CSF culture with a negative CRP. Of the charts reviewed 171 patients had elevated CRP levels in the absence of blood, CSF, and urine culture growth.
CSF analysis seemed to depend on the growing organism (Table 2). Patients with Klebsiella Pneumonia had normal cell count, protein, and glucose. While abnormal values of all the three elements were associated with E. coli, Pseudomonas, and staph; these patients had significantly low glucose levels along with high protein and pleocytosis. Patients who grew Group B strep had variable results: All had pleocytosis, low glucose, and high protein except for one patient who had a completely normal analysis, and another who had pleocytosis with high protein but normal glucose level.
| Table 2: CSF analysis of patients with Positive bacterial CSF cultures | |||||
| CSF Culture | CSF WBC Count x10(3)/mcL | CSF RBC Count x10(12)/L | CSF Protein g/L | CSF Glucose mmol/L | |
| 1 | Escherichia coli | 3490 | 660 | 3.98 | <0.2 |
| 2 | Escherichia coli | 24620 | 10000 | 4.3 | <0.2 |
| 3 | Escherichia coli | 0 | 0 | 5.8 | 0.2 |
| 4 | staphylococcus aureus | 570 | 11040 | 8.3 | 0.6 |
| 5 | staphylococcus epidermidis | 200 | 25 | 9.68 | <0.2 |
| 6 | Klebsiella Pneumoniae | 37 | 40277 | ||
| 7 | Klebsiella Pneumoniae | 2 | 1 | 0.41 | 3.6 |
| 8 | Klebsiella Pneumoniae | 4 | 900 | 0.57 | 4 |
| 9 | Pseudomonas aeruginosa | 5700 | 14 | 6.89 | <0.6 |
| 10 | Streptococcus agalactiae (Group B) | 151 | 50 | 23.8 | 2.5 |
| 11 | Streptococcus agalactiae (Group B) | 2910 | 485 | 11.89 | <0.2 |
| 12 | Streptococcus agalactiae (Group B) | 980 | 360 | 3.45 | 0.8 |
| 13 | Streptococcus agalactiae (Group B) | 5460 | 6900 | 2.87 | 0.2 |
| 14 | Streptococcus agalactiae (Group B) | 4 | 3 | 0.65 | 2.9 |
| 15 | Streptococcus agalactiae (Group B) | 2585 | 13000 | 2.93 | 0.6 |
| 16 | Streptococcus pneumoniae | 21 | 17 | 1.07 | 3.9 |
In those patients with viral meningitis, all 11 patients grew Enterovirus, and none had positive bacterial growth in the blood cultures. Unfortunately, blood samples were not sent for viral testing so a correlation between viral growth in CSF and blood samples could not be made. Both WBC count and CRP level were all within normal for these patients except for one who had a mild elevation of CRP of 46. The CSF analyses were all within normal limits except for three patients who had significant pleocytosis alone in the absence of traumatic tap (Table 3). The average age of patients in this group was 5.7 weeks, with two to three weeks of age being the most frequently affected.
| Table 3: CSF analysis of patients diagnosed with viral meningitis. | |||||
| CSF Culture | CSF WBC Count x10(3)/mcL | CSF RBC Count x10(12)/L | CSF Protein g/L | CSF Glucose mmol/L | |
| 1 | Enterovirus | 134 | 25680 | 0.6 | 2.1 |
| 2 | Enterovirus | 1 | <1 | 0.44 | 2.6 |
| 3 | Enterovirus | 2 | <1 | 0.41 | 2.5 |
| 4 | Enterovirus | 42 | 28 | 1.05 | 2.2 |
| 5 | Enterovirus | 474 | 5750 | 1.32 | 2.5 |
| 6 | Enterovirus | 2 | 21360 | 1.69 | 3.4 |
| 7 | Enterovirus | 38 | <1 | 0.42 | 2.5 |
| 8 | Enterovirus | 4 | <1 | 0.53 | 3.3 |
| 9 | Enterovirus | 19 | 16000 | 1.12 | 2.8 |
| 10 | Enterovirus | 1027 | 4 | 0.72 | 2.7 |
| 11 | Enterovirus | 397 | <1 | 0.59 | 2 |
Based on our study, 33% of our patients developed meningitis due to different organisms in the absence of positive blood culture. Similarly, unfortunately, CBC, CRP, and even CSF analysis seem to have limited predictive values leaving CSF culture to be the sole golden standard in ruling in or ruling out bacterial meningitis in this vulnerable age group.
The only correlation between those patients who had a positive CSF culture and negative blood culture was that all except one were full-term infants who developed meningitis in the first month of life. Also, all patients had normal WBC counts.
Garges, et al. reached similar conclusions in their study that was conducted in 2006. Out of 9111 patients, 92 had true positive CSF culture; and of those 35 had no blood culture growth (38%). The study went further to analyze CSF WBC, Glucose, and protein levels and found them not to be very predictive of true infection.
Similarly, Harmony, et al. found that CSF analysis was unreliable in predicting bacterial meningitis as many of the patients had completely normal CSF analyses, and other patients who had no CSF bacterial or viral growths had pleocytosis for unexplained reasons. The reliability of the CRP and WBC counts in predicting meningitis were also weak as many patients had either normal counts and levels of CRP or mildly elevated CRP levels not representative of the severity of the infection.
In those patients with bacterial meningitis, 77% were affected during their first month of life solidifying the finding of an earlier study by Thigpen, et al. in 2011 which concluded that the first month of life was when infants were most prone to develop meningitis.
Although this study only focused on the results of blood and CSF cultures, newer methods of bacterial detection through PCR testing is available and is proven to be more accurate in the detection of bacteria [6,7]. This testing was unfortunately not available in our facility, but its implementation may detect higher numbers of positive CSF or blood cultures [6,7].
Infants from zero to 90 days frequently suffer from febrile illnesses, most of which are simple. However, obtaining CSF samples for culture remains the only reliable way to evaluate bacterial meningitis mainly in the first month of life. Negative blood cultures and normal CBC and CRP may not be reliable enough to completely rule out the presence of meningitis. The same applies to CSF analysis which implies that pediatricians should continue empirical antibiotics until the final culture result is available in this age group.
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, Craig AS, Schaffner W, Thomas A, Lewis MM, Scallan E, Schuchat A; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011 May 26;364(21):2016-25. doi: 10.1056/NEJMoa1005384. PMID: 21612470.
- Harvey D, Holt DE, Bedford H. Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Semin Perinatol. 1999 Jun;23(3):218-25. doi: 10.1016/s0146-0005(99)80066-4. PMID: 10405191.
- Garges HP, Moody MA, Cotten CM, Smith PB, Tiffany KF, Lenfestey R, Li JS, Fowler VG Jr, Benjamin DK Jr. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006 Apr;117(4):1094-100. doi: 10.1542/peds.2005-1132. PMID: 16585303.
- Wiswell TE, Baumgart S, Gannon CM, Spitzer AR. No lumbar puncture in the evaluation for early neonatal sepsis: will meningitis be missed? Pediatrics. 1995 Jun;95(6):803-6. PMID: 7761203.
- Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol. 2015 Mar;42(1):29-45, vii-viii. doi: 10.1016/j.clp.2014.10.004. Epub 2014 Dec 6. PMID: 25677995; PMCID: PMC4332563.
- Griffiths MJ, McGill F, Solomon T. Management of acute meningitis. Clin Med (Lond). 2018 Mar;18(2):164-169. doi: 10.7861/clinmedicine.18-2-164. PMID: 29626023; PMCID: PMC6303447.
- Khater WS, Elabd SH. Identification of Common Bacterial Pathogens Causing Meningitis in Culture-Negative Cerebrospinal Fluid Samples Using Real-Time Polymerase Chain Reaction. Int J Microbiol. 2016;2016:4197187. doi: 10.1155/2016/4197187. Epub 2016 Aug 1. PMID: 27563310; PMCID: PMC4983665.