More Information
Submitted: August 24, 2022 | Approved: September 01, 2022 | Published: September 02, 2022
How to cite this article: Idrissi SA, Elbazi K, Boucetta BA, Gennouni M, El Anssari S, et al. Post-vaccine immunity against hepatitis B in Moroccan children. J Adv Pediatr Child Health. 2022; 5: 028-032.
DOI: 10.29328/journal.japch.1001049
Copyright License: © 2022 Idrissi SA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hepatitis B; Vaccine; Immunization; Anti-HBs antibodies
Post-vaccine immunity against hepatitis B in Moroccan children
SA Idrissi1*, K Elbazi1, B Ait Boucetta1, M Gennouni2, S El Anssari1, I Brahim1, R Hazime1 and B Admou1,3
1Laboratory of Immunology, University Hospital Mohammed VI, Marrakech, Morocco
2Hassan First University of Settat, Laboratory of Health Sciences and Technologies, Morocco
3Biosciences Research Laboratory, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech, Morocco
*Address for Correspondence: SA Idrissi, Laboratory of Immunology, University Hospital Mohammed VI, Marrakech, Morocco, Email: [email protected]
Background: Hepatitis B is a major public health issue worldwide. Immunization of infants against this disease has been effective in Morocco since 1999. However, evaluation of post-vaccination response is rarely performed in our setting. The purpose of this study was to evaluate immunity against HBV in fully vaccinated children in the city of Marrakech in Morocco and to investigate the factors influencing the level of post-vaccination immunity.
Methods: A descriptive cross-sectional study was conducted on fully vaccinated children who have medical and vaccination records, from three primary healthcare centers in Marrakech. Children with anti-HBs antibody levels between 10 and 100 IU/L were considered moderately immune, and those with antibody levels above 100 IU/L as highly immune, while those with antibody levels below 10 IU/L were considered non-immune.
Results: Of the 123 children recruited, 114 (92.7%) had protective anti-HBs antibody titers, of which 37 (30%) were moderately immunized and 77 (62.7%) were highly immunized, and nine (7.3%) were non-immune. Age, birth weight, vaccine type, and time since the previous dose have all been significantly associated with the degree of post-vaccination immunity. Anti-HBs antibody levels were not significantly related to factors potentially linked to post-vaccination non-response, such as chronic disease, immunosuppressive medication and others.
Conclusion: Our findings denote that the HBV vaccine used in The Moroccan Expanded Program on Immunization (EPI) is effective against HBV. Nevertheless, in non-responders, corrective actions such as re-vaccination and monitoring of post-vaccination anti-HBs antibody levels should be implemented.
Hepatitis B Virus (HBV) is the leading cause of acute and chronic liver disease and is associated with morbidity and mortality worldwide with 887 000 deaths in 2015 [1,2]. Vertical and horizontal early childhood transmission are the main routes of HBV transmission and are responsible for most chronic infections [3]. Universal hepatitis B immunization at birth and in infancy is the key strategy for the global elimination of HBV infection. The world’s first HBV vaccination program for infants was launched in Taiwan in July 1984 [4].
The World Health Organization (WHO) established a target for introducing HBV vaccination into national immunization programs by 1995 for countries with an HBV carrier rate of ≥ 8% and by 1997 for all countries [5], and advocated for the administration of a birth dose to all newborns within 24 hours from birth by 2009 [6]. Vaccination against viral hepatitis B was introduced in Morocco in July 1999 [7] and is now mandatory.
Vaccination strategy against the hepatitis B epidemic has become the most cost-effective public health measure implemented so far, to fight mortality and morbidity linked to this epidemic [8,9]. According to WHO data, Morocco purportedly had an intermediate prevalence of 2% - 7% prior to the introduction of the hepatitis B vaccine into the National Immunization Program. In 2020, HBV prevalence was about 1.79% of the general population [10]. Vaccination is therefore considered the most efficient way to control hepatitis B, and immunization of newborns is necessary to prevent perinatal HBV transmission. The purpose of this study was to evaluate immunity against HBV in fully vaccinated children in the city of Marrakech in Morocco and to investigate the factors influencing the level of post-vaccination immunity.
A descriptive, prospective, cross-sectional study was conducted on 126 children recruited at the level of three Basic Healthcare Centers (BHC) in the city of Marrakech. These centers were chosen randomly out of the 51 centers located in the different regions of the city: one center in the north, one center in the West, and one in the South. The 4th center in the East region was excluded due to non-compliance with the eligibility criteria: the availability of vaccination registers containing socio-demographic data (age, sex, weight, socio-economic level, and level of education), methods of vaccine administration (vaccine type, batch number, expiration date, cold chain, injection site, and doses and quantities administered), and clinical data that may influence the efficacy of immunizations (pathological conditions, drug intake, etc.). Children vaccinated before 2012 received three doses of the monovalent vaccine while those vaccinated after 2012 benefited from a new vaccination protocol based on one dose of monovalent vaccine and two doses of pentavalent vaccine. Only children who received three doses of the vaccine on a vaccination card were included. Consent was clearly provided by the parents to collect the data.
We used Serum Separating Tubes (SST) to collect each participant’s blood samples which consisted of 4 mL for population members above two years old and 2 mL for children two years old or younger. The Faculty of Medicine and University Hospital Center of Marrakech Laboratory of Immunology received the labeled and numbered collection tubes of serum where they centrifuged and stored the serum in freezing tubes at - 20 °C to wait for analysis. We established a detailed laboratory testing protocol before testing. We used an Enzyme-Linked Immunosorbent Assay technique (Monalisa, anti-HBS+ tm, Bio-rad, France) to determine the number of anti-HBS antibodies and estimated the detection threshold for anti-HBS Abs to be 10 IU/L.
Anti-HBs are the only easily measurable correlation of vaccine-induced protection using serologic assays. An anti-HB level of 10 IU/L or more is considered a reliable marker of protection against infection and protects against both acute and chronic infection for decades [11]. Moreover, patients with anti-HBs levels between 10 and 100 IU/L are considered moderately immune, whereas those with anti-HBs levels> 100 IU/L are considered highly immune [12].
Data collected from exploitation sheets were analyzed using SPSS software- Statistical Package for Social Sciences Software for Windows (SPSS version 25.0, SPSS Inc., Chicago, IL, USA). The difference is considered significant if p < 0.05. The comparison between the categorical variables was made by the chi-square test or the Fisher test.
Characteristics of the study population
126 children were enrolled in the study. The average age of the participants was 4.6 ± 3.1 years, with extremes ranging from 6 months to 16 years. Males were slightly more represented (50.4%). All of the patients were from Marrakech, with 113 hailing from urban areas. The median socioeconomic level accounted for 56.9% of the cases. All participants received the vaccine at birth, and only three were born to HBV carrier mothers. The average birth weight was 3.22 (± 0.65) kg.
Tables 1,2 summarize the sociodemographic and clinical characteristics of the participants respectively.
| Table 1: Sociodemographic characteristics of children included in the study. | ||
| Variable | Number of participants | Percentage (%) |
Gender:
|
62 61 |
50.4% 49.6% |
Age:
|
32 46 45 |
26% 37.4% 36.6% |
Socio-economic level:
|
53 70 |
43.1% 56.9% |
Area:
|
113 10 |
91.9% 8% |
Medical insurance status:
|
13 110 |
10.6% 89.4% |
| Table 2: Clinical characteristics of children included in this study. | ||
| Parameters | Number of participants | Percentage (%) |
Hepatitis B carrier mother
|
3 123 |
2.4% 97.6% |
Duration since the last dose of vaccine
|
49 74 |
40% 60% |
Birthweight:
|
13 95 15 |
10.6% 77.2% 12.2% |
Actual weight:
|
85 14 24 |
69.1% 11.4% 19.5% |
Documented past illnesses:
|
3 5 2 8 |
2.4% 4% 1.6% 6.5% |
| Chronic illnesses a | 4 | 3.2% |
| Corticosteroid immunosuppressive treatment | 3 | 2.4% |
Breastfeeding status:
|
65 21 37 |
52.8% 17.1% 30.1% |
| Second-hand smoking | 45 | 36.6% |
Type of vaccine:
|
93 30 | 75.6% 24.4% |
Vaccine injection site:
|
15 8 |
93.5% 6.5% |
| Compliance with vaccination schedule: | 79 | 64% |
| Side effects d | 49 | 39.8% |
| a: Asthma, chronic diarrhea b: before 2012, c: after 2012 d: Side effects such as fever, fatigue, swelling, redness, injection site pain, myalgia |
||
Prevalence of adequate immunization
Participant’s anti-HBs antibody titers ranged from 5.0 to 950.0 IU/L, with a median (25th, 75th percentile) titer of 133.4 (49.2, 434.1) IU/L and an average of 314.3 ± 340 IU/L. Of the 123 participants, 114 had anti-HBs titers > 10.0 IU/L giving a prevalence of 92.7%. Moreover, 37 children were moderately immune and 77 were extremely immune.
Factors associated with an adequate immune response
Antibody titer was associated with duration since the last dose of vaccine (Figure 1), birth weight (Figure 2), and type of vaccine administrated. Variables such as sex, age at first dose, type of breastfeeding, chronic illnesses, and others did not significantly influence antibody titer. Table 3 summarizes the variation of anti-HBs titer according to these different variables.
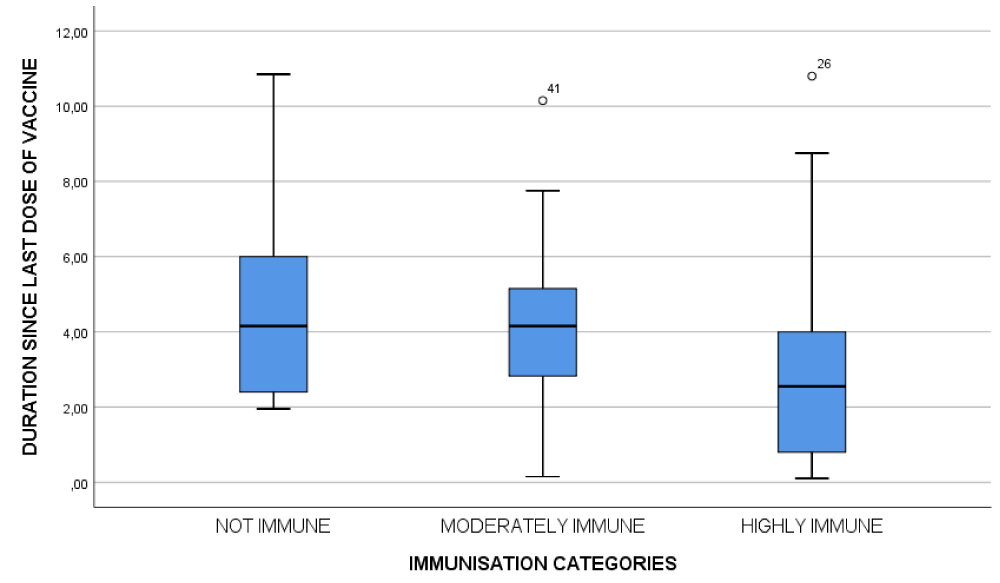
Figure 1: Correlation between immunization levels and duration since the last dose of vaccine.
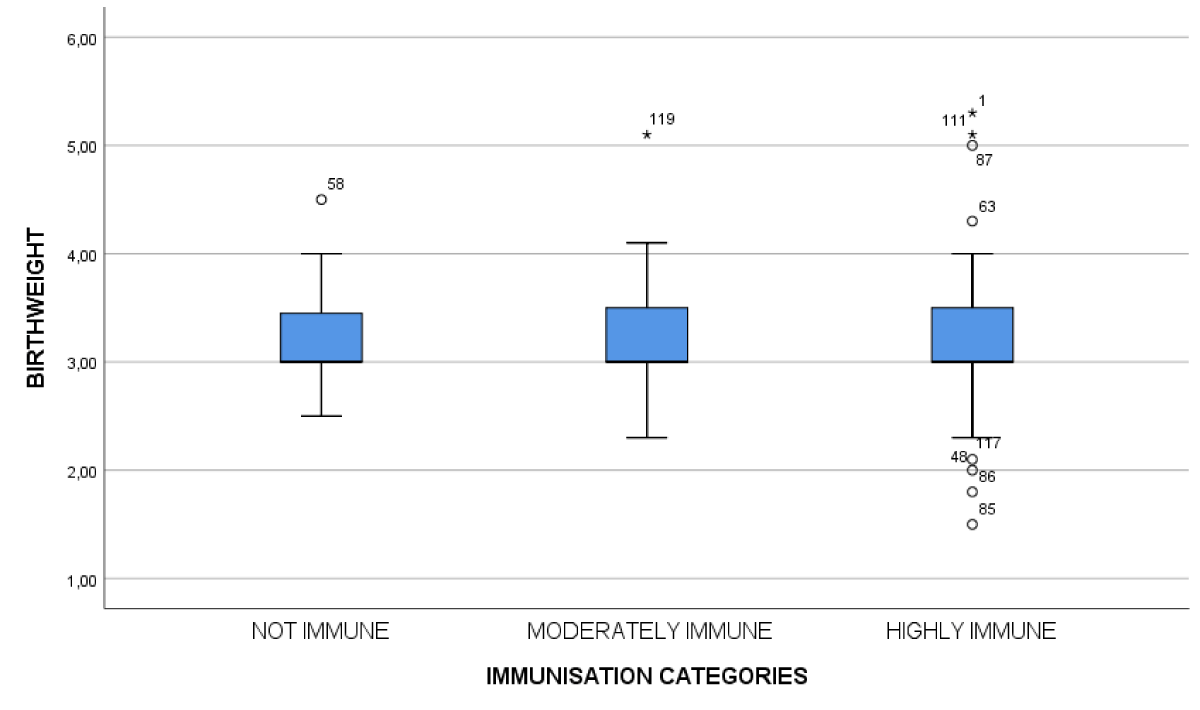
Figure 2: Correlation between immunization levels and birth weight.
| Table 3: Variation of anti-HBs titer according to different parameters. | ||||
| Variables | Not immune | Moderately immune | Highly immune | p value |
Gender:
|
72.7% 27.3% |
37.1% 62.9% |
53.2% 46.8% |
0.086 |
Age:
|
27.3% 18.1% 54.6% |
27.2% 49.4% 23.4% |
22.9% 17.1% 60% |
< 0.001 |
Habitat:
|
90.9% 9.1% |
91.4% 8.6% |
92.2% 7.8% |
0.983 |
Socio-economic level:
|
45.5% 54.5% |
45.7% 54.3% |
42.1% 57.9% |
0.929 |
Medical insurance status:
|
9.1% 90.9% |
11.4% 88.6% |
10.4% 89.6% |
0.933 |
Hepatitis B carrier mother:
|
0% 100% |
5.7% 94.3% |
1.3% 98.7% |
0.321 |
Duration since the last dose of vaccine:
|
9% 91% |
19% 81% |
52% 48% |
< 0.001 |
Birthweight:
|
0% 90.9% 9.1% |
6.9% 82.8% 10.3% |
7.9% 81% 11.1% |
< 0.001 |
Actual weight:
|
63.3% 18.2% 18.2% |
60% 17.1% 22.9% |
70.4% 7.8% 18.2% |
0.5 |
Breastfeeding status:
|
63.6% 27.3% 9.1% |
57.1% 11.4% 31.4% |
49.4% 18.2% 32.5% |
0.448 |
Documented past illnesses:
|
0% 100% |
14.3% 85.7% |
16.9% 83.1% |
0.333 |
Chronic illnesses:
|
0% 100% |
2.9% 97.1% |
3.9% 96.1% |
0.783 |
Corticosteroid immunosuppressive treatment:
|
0% 100% |
2.9% 97.1% |
1.3% 98.7% |
0.754 |
Second-hand smoking:
|
45.5% 54.5% |
35.1% 62.9% |
35.1% 64.9% |
0.797 |
Vaccine injection site:
|
100% 0% |
97.1% 2.9% |
90.9% 9.1% |
0.305 |
Type of vaccine:
|
92% 8% |
70% 30% |
75% 25% |
0.04 |
Compliance with vaccination schedule:
|
16.7% 83.3% |
60.4% 39.6% |
75% 25% |
0.313 |
A relational analysis between duration since the last dose of vaccine and anti-HBs titer showed a negative correlation, meaning antibody titers had decreased as duration increased (p < 0.01). We also determined that the lower the birth weight, the lower the titer; there was a statistically significant association between vaccination immunity and birth weight (p < 0.01). Furthermore, we noted a significant difference between the group of children receiving the monovalent vaccine alone and those receiving the combined pentavalent vaccine (p = 0.04).
Last but not least, and according to our survey, the cold chain was respected both during the transport and storage period, and the vaccines were administered under the required expiry dates. The rules of asepsis and hygiene were respected during vaccination at the various health structures.
The majority of the participants in this study (92.7%) had an adequate immune response to HBV following three doses of vaccination received during the routine EPI. Non-responders exhibited no unusual findings. This result was slightly lower than the WHO’s recommended global level of > 95% [13] but was consistent with several investigations in Africa [14-16]. However, prior investigations from Senegal, Ethiopia, Ghana, and Nigeria [17-20], found lower seroprotection rates. The disparities in seroprotection rates could be explained by a variety of reasons, including the genetic characteristics of participants, the age groups studied, methodological discrepancies, vaccine type, dosage, and storage conditions.
There was no significant difference in seroprotection levels between girls and boys (p = 0.086), which is consistent with some research [15] but differs from others in which females responded better to the vaccine than males [21] and/or vice versa [19,22]. Younger age was found to be significantly associated with high levels of anti-HBs. As a result, older children make up the majority of individuals who lack protective antibody levels. More so, a relational analysis between duration since the last dose of vaccine and anti-HBs titer showed a negative correlation. These findings were similar to several previous research [15,21-23]. Thus, as children aged, the antibody levels produced following vaccination waned.
Birth weight was the second major factor influencing the level of post-vaccination immunity, as reported by other authors as well [15,16,18,19]. The low birth weight that is traditional during prematurity is accompanied by a fineness of the skin and a lack of muscle mass. This situation could hinder the administration or resorption of vaccines, the intramuscular and subcutaneous routes being the most used for vaccination [16]. Others consider obesity to be one of the factors decreasing this immune response [12,24]. The type of vaccine and the number of doses received were also associated with the degree of post-vaccination immunity. Children who received the pentavalent vaccine had a better immunization rate than those who received the monovalent vaccine (p = 0.04). Similar results were found in Burkina Fasso [16] and Taiwan [25].
Concurrent chronic disease and immunodepression are the most common reasons for the non-attainment of seroprotection amongst vaccinated children. The low prevalence of these factors in the study population could partly explain. However, more research is therefore warranted to draw meaningful conclusions. Moreover, 64% of the participants received the hepatitis vaccines according to the recommended schedule. The high incidence of vaccine response can also be explained by the promptness with which vaccines are administered. However, there was no association between anti-HBs reaction and vaccine delivery time. This finding could imply that children who have missed a dose of the hepatitis vaccine should be urged to catch up at the next available opportunity. More evidence, however, is required to fully comprehend this.
Overall, the majority of the participants showed protective anti-HBs titers and followed the EPI protocol. Age, birth weight, vaccine type, and time since the previous dose have all been associated with the degree of post-vaccination immunity. The anti-HBs titer levels were not significantly connected to the other features of the individuals. There were no variations in the investigated risk factors linked with non-response, implying the presence of a previously unreported genetic component.
Strengths and limitations
As far as we are aware, this study is the first to assess the level of immunity following anti-HBV vaccination in a broad pediatric population in Morocco.
The study’s drawbacks, on the other hand, are its limited sample size and selection bias as a result of its singular emphasis. In order to provide better findings, especially in non-immune patients, it is believed that a larger sample size obtained from different Moroccan cities and a focus on potential risk factors that may affect HBV immunization are necessary.
Chronic viral hepatitis B remains a significant public health problem. Its treatment must remain primarily preventive by vaccination. Vaccination against HBV has demonstrated its effectiveness for several years since its marketing and its recommendation by the WHO. This study has demonstrated the excellent immunization offered by this vaccination. 92% of vaccinated children acquired an immunization rate considered protective to varying degrees. However, those who are not immunized must first and foremost be investigated to substantiate the causes of non-immunization and to undertake corrective measures including re-vaccination combined with monitoring of the post-vaccination anti-HBs antibody levels.
We thank all our colleagues and staff members from the immunology department of University Hospital Mohammed VI who provided insight and technical expertise that greatly assisted the research.
- Sbai A, Baha W, Ougabrai H, Allalia T, Dersi N, Lazaar F, Ennaji MM, Benjouad A, El Malki A, Hassar M, Benani A. Prévalence de l'infection par le virus de l'hépatite B et l'évaluation des facteurs de risque au Maroc [Hepatitis B prevalence and risk factors in Morocco]. Pathol Biol (Paris). 2012 Oct;60(5):e65-9. French. doi: 10.1016/j.patbio.2011.06.001. Epub 2011 Aug 3. PMID: 21816547.
- WHO Global hepatitis report. Geneva: Health World Organization 2017;
- Indolfi G, Easterbrook P, Dusheiko G, Siberry G, Chang MH, Thorne C. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019; 4(6):466–76. http://dx.doi.org/10.1016/S2468-1253(19)30042-1
- Ni YH, Chen DS. Hepatitis B vaccination in children: the Taiwan experience. Pathol Biol (Paris). 2010 Aug;58(4):296-300. doi: 10.1016/j.patbio.2009.11.002. Epub 2010 Jan 29. PMID: 20116181.
- Komatsu H. Hepatitis B virus: where do we stand and what is the next step for eradication? World J Gastroenterol. 2014 Jul 21;20(27):8998-9016. doi: 10.3748/wjg.v20.i27.8998. PMID: 25083074; PMCID: PMC4112872.
- Álvarez AMR, Pérez-Vilar S, Pacis-Tirso C, Contreras M, Omeiri N El, Ruiz-Matus C. Progress in vaccination towards hepatitis B control and elimination in the Region of the Americas. BMC Public Heal. 2017; 17(1):1–10. https://link.springer.com/articles/10.1186/s12889-017-4227-6
- Lamdouar Bouazzaoui N. Évolution du calendrier vaccinal au Maroc. Bull Acad Natl Med. 2006; Apr 1:190(4–5):1017–33.
- Lok ASF. Hepatitis B: 50 years after the discovery of Australia antigen. Viral Hepat J 2016 Jan 1; 23(1):5–14. https://onlinelibrary.wiley.com/doi/full/10.1111/jvh.12444
- Al Awaidy ST, Bawikar SP, Al Busaidy SS, Al Mahrouqi S, Al Baqlani S, Al Obaidani I, Alexander J, Patel MK. Progress toward elimination of hepatitis B virus transmission in Oman: impact of hepatitis B vaccination. Am J Trop Med Hyg. 2013 Oct;89(4):811-5. doi: 10.4269/ajtmh.13-0333. Epub 2013 Aug 19. PMID: 23958910; PMCID: PMC3795119.
- Madihi S, Syed H, Lazar F, Zyad A, Benani A. A Systematic Review of the Current Hepatitis B Viral Infection and Hepatocellular Carcinoma Situation in Mediterranean Countries. Biomed Res Int. 2020 Jun 10;2020:7027169. doi: 10.1155/2020/7027169. PMID: 32626758; PMCID: PMC7305551.
- Van Damme P, Ward JW, Shouval D, Zanetti A. Hepatitis B Vaccines. Plotkin’s Vaccines. 2018 Jan 1; 342-374.e17. https://linkinghub.elsevier.com/retrieve/pii/B9780323357616000250
- Michel ML, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol (Paris). 2010 Aug;58(4):288-95. doi: 10.1016/j.patbio.2010.01.006. Epub 2010 Apr 10. PMID: 20382485.
- World Health Organization. Global hepatitis report, 2017. Geneva. Glob Hepat report, 2017; 7-20.
- Haban H, Benchekroun S, Sadeq M, Benjouad A, Amzazi S, Oumzil H, Elharti E. Assessment of the HBV vaccine response in a group of HIV-infected children in Morocco. BMC Public Health. 2017 Sep 29;17(1):752. doi: 10.1186/s12889-017-4776-8. PMID: 28962610; PMCID: PMC5622525.
- Anutebeh EN, Tatah L, Feteh VF, Aroke D, Assob JCN, Choukem SP. Immune response to hepatitis B vaccine following complete immunization of children attending two regional hospitals in the Southwest region of Cameroon: a cross sectional study. BMC Infect Dis. 2021;21(1):1–8. https://doi.org/10.1186/s12879-021-06913-y
- Kissou SA, Sidibé K, Bazie W, Sourabié Y, Cessouma KR, Ouedraogo AS. Post-Vaccine Immunity against Hepatitis B in Burkina Faso children. 2018. www.symbiosisonlinepublishing.com
- Rey-Cuille MA, Seck A, Njouom R, Chartier L, Sow HD, Mamadou, Ka AS, Njankouo M, Rousset D, Giles-Vernick T, Unal G, Sire JM, Garin B, Simon F, Vray M. Low immune response to hepatitis B vaccine among children in Dakar, Senegal. PLoS One. 2012;7(5):e38153. doi: 10.1371/journal.pone.0038153. Epub 2012 May 30. PMID: 22666468; PMCID: PMC3364238.
- Teshome S. Antibody level against HBV after Hepatitis B vaccination and Sero-prevalence of HBV in children in Addis Ababa, Ethiopia. 2017;
- Apiung T, Ndanu TA, Mingle JA, Sagoe KW. Hepatitis B virus surface antigen and antibody markers in children at a major paediatric hospital after the pentavalent DTP-HBV-Hib vaccination. Ghana Med J. 2017 Mar;51(1):13-19. doi: 10.4314/gmj.v51i1.3. PMID: 28959067; PMCID: PMC5611951.
- Odusanya OO, Alufohai E, Meurice FP, Ahonkhai VI. Five-year post vaccination efficacy of hepatitis B vaccine in rural Nigeria. Hum Vaccin. 2011 Jun;7(6):625-9. doi: 10.4161/hv.7.6.14990. Epub 2011 Jun 1. PMID: 21508678.
- Kissou S, Sidibé K, Sourabié Y, Cessouma K, Ouedraogo A, Sawadogo A. Post-Vaccine Immunity against Hepatitis B in Burkina Faso children. J Gastroenterol Pancreatol Liver Disord. 2018; 6(1):1–4.
- Van der Sande MAB, Waight PA, Mendy M, Zaman S, Kaye S, Sam O, et al. Long-Term Protection against HBV Chronic Carriage of Gambian Adolescents Vaccinated in Infancy and Immune Response in HBV Booster Trial in Adolescence. PLoS One. 2007 Aug 15; 2(8):e753. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0000753
- Van Damme P. Long-term Protection After Hepatitis B Vaccine. J Infect Dis. 2016; 214(1):1–3. https://academic.oup.com/jid/article/214/1/1/2469743
- Hanslik T, Boëlle PY. L'évaluation du rapport risque/bénéfice des stratégies de vaccination [Benefit-risk assessment of vaccination strategies]. Med Sci (Paris). 2007 Apr;23(4):391-8. French. doi: 10.1051/medsci/2007234391. PMID: 17433229.
- Mahoney FJ. Update on diagnosis, management, and prevention of hepatitis B virus infection. Clin Microbiol Rev. 1999 Apr;12(2):351-66. doi: 10.1128/CMR.12.2.351. PMID: 10194463; PMCID: PMC88921.