More Information
Submitted: December 02, 2022 | Approved: January 18, 2023 | Published: January 19, 2023
How to cite this article: Bisht N, Garg AP. Health management using probiotics. J Adv Pediatr Child Health. 2023; 6: 001-006.
DOI: 10.29328/journal.japch.1001053
Copyright License: © 2023 Bisht N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Health management using probiotics
Neha Bisht and Amar P Garg*
School of Biological Engineering & Life Sciences, Shobhit Institute of Engineering & Technology (NAAC ‘A’ Grade Accredited Deemed-to-be-University) Modipuram, NH-58, Meerut 250110, India
*Address for Correspondence: Dr. Amar P Garg, Professor, School of Biological Engineering & Life Sciences, Shobhit Institute of Engineering & Technology (NAAC ‘A’ Grade Accredited Deemed-to-be-University) Modipuram, NH-58, Meerut 250110, India, Email: [email protected]
‘Let food be thy medicine and medicine be thy food’, the age-old quote by Hippocrates is evidently proved in today’s life management as probiotics have become a valuable part of human day-to-day life [1]. Eating habits, mother’s milk and quality of foods are mainly responsible for overall health status. Humans consumed food with several live microorganisms in earlier times. However, the concept of hygiene improved the criteria for eating clean food with little live bacteria. The intake of fermented foods has significantly decreased due to the Western diet hence reducing the number of probiotic organisms to which human ancestors were exposed [2]. The Western diet’s declining probiotic content automatically increases cases of malignancies, allergies, obesity, heart disease, and autoimmune disorders [3]. The use of probiotics influences human physiology by modulation of gut microbiota and mucosal immunity [4]. US FDA recommended probiotic strains. Various Studies have shown that lactose-intolerant individuals digest dairy products that have been fermented are superior to milk when compared in size [5-8]. L. delbrueckii and S. thermophiles used as a starter culture enhance lactose digestion and get rid of lactose intolerance symptoms when yogurt is made [9]. The fermented dairy products were less likely to cause gastric upsets in various ways as follows.
1) Fermented products contain 4% - 6% of lactose as a result of microbial digestion during fermentation. 2) Live bacteria found in fermented foods operate as bacterial lactase in the gut lumen in vivo, facilitating lactose breakdown and reducing lactose intolerance [10-12]. 3) Slow gastric emptying of the fermented milk product due to high viscosity as compared to milk and slower transit time of yogurt may permit the residual intestinal lactase and yogurt bacteria to digest the lactose [13].
Cholesterol assimilation
Due to the high levels of cholesterol in people’s bloodstream, the risk of coronary heart disease was rising daily [14-16]. Shaper, et al. 1963 [17] and Mann, 1974 [18] for the first time observed that drinking fermented milk containing Lactobacilli claimed to control hypocholesterolemic effects among people. Since then, a number of studies had also revealed that consuming fermented dairy products lowers the chance of developing high cholesterol [19-23]. Several mechanisms had been postulated regarding the effects of hypocholesterolemic induced by lactic acid bacteria. Assimilation of cholesterol through cell growth or attachment to the cell surface was one of the mechanisms carried by lactic acid bacteria [24,25]. The second method involves the bile salts deconjugation due to the enzyme bile salt hydrolase (BSH), which hydrolyzes conjugated bile salts of taurine and glycine into residues of amino acid and free bile salts (bile acids). As a result of being less soluble, these deconjugated bile salts were expelled in the feces and replenished by fresh bile salts, which the body produced from cholesterol. The body removes more cholesterol when more bile salts are secreted [16,26]. When cultivated in broth medium supplemented with 0.2% bile salts, studies showed that Lactobacillus acidophilus PI06 appeared to have an active bile salt hydrolase that eliminated cholesterol from the body roughly 29.02% to 45.3%. [27,28] (Figure 1).
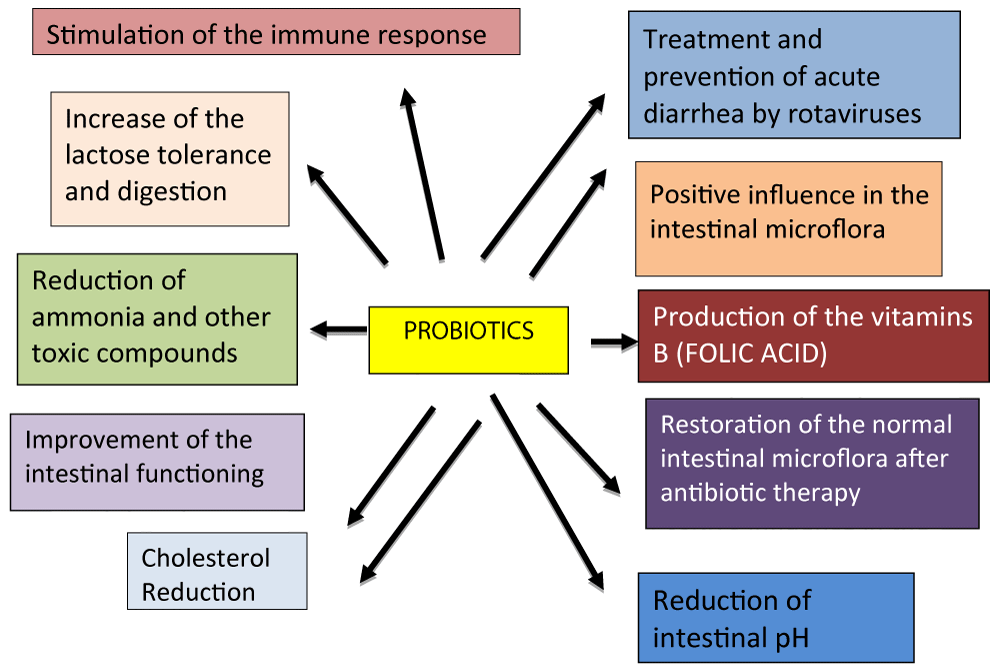
Figure 1: Beneficial effects of probiotics on human health.
L. Plantarum S4-1 isolated from Chinese sauerkraut was examined in vivo in a 2013 study by Yu, et al. [23] The strain was able to lower mice’s serum cholesterol levels when fed fermented milk containing it. Kumar, et al. 2011 [29] stated that the strains of L. Plantarum such as Lp91 and Lp21 showed a potential BSH activity in rats resulting in the lowering of cholesterol through cholesterol assimilation and cholesterol co-precipitation. Another potential method for decreasing cholesterol was propionate resulting from the end product of short-chain fatty acid. Studies conducted by Liong and Shah [22] reported that short-chain fatty acids played a role in the altered lipid metabolism that resulted in a drop in the body’s serum cholesterol level (Table 1).
| Table 1: Recommended doses of Lactobacilli (Bhadoria and Mahapatra, 2011). | ||
| S.No | Strains | The effective dose in cfu/d |
| 1. | L. caseishirota | 6.5 × 109 |
| 2. | L. rhamnosus GG | 109 × 1010 |
| 3. | L. Plantarum 299 v | 5 × 108 |
| 4. | L. acidophilus NCFB 1748 | 3 × 1011 |
| 5. | L. reuteri | 1 × 108 -1011 |
| 6. | L. rhamnosus DSM 6594 | 16 × 109 |
Immune response
Parvez, et al. 2006 [30] stated that the immune system was an extremely complex system that involves cell-based as well as antibody-based responses against potential infectious agents. The modification in the host “immunity” is the most frequently cited advantage of probiotic ingestion. The use of probiotics has an effect on the immune system by enhancing both specific and non-specific immune responses, according to evidence from in vitro research utilizing animal models and humans. These reactions were thought to be mediated by activating macrophages, up-regulating cytokines, up-regulating natural killer (NK) cell activity and up-regulating immunoglobulin levels [31,32]. Kaila, et al. 1992 [33] stated that the infants with acute rotaviral diarrhea had been given Lactobacillus rhamnosus strain GG (LGG) resulted in increased immunoglobulins like (IgA), (IgG) and (IgM) resulting in shortened duration of gastroenteritis symptoms. Studies showed that probiotics also stimulate the production of systemic and mucosal IgA in humans [33-35]. Various studies reported that probiotics improve the intestinal mucosa’s ability to act as an immune barrier by secreting IgA. By recognizing toll-like receptor 2 and toll-like receptor 9, probiotic bacteria’s cell walls components, such as lipoteichoic acids, peptide glycans and DNA motifs, caused modulatory effects [36,37]. Intake of fermented milk with L. johnsoniior Bifidobacterium bifidum increases phagocytosis of E. coli as well as increased serum IgA response to Salmonella typhi [34-42]. Several studies had showed that probiotics improve immune responses to immunization with poliovirus. A live attenuated poliovirus vaccination produced much stronger virus-neutralizing antibody responses (mostly IgA) in yogurt containing L. rhamnosus and L. paracasei than in uncontaminated yogurt. [43]. Schiffrin, et al. in 1997 [38] reported the incredible potential of healthy human peripheral blood leucocytes to phagocytose when given fermented milk supplemented with Lactobacillus johnsonii Lai or Bifidobacterium lactis Bbl2. Studies on animals showed that the phagocytic cell’s improved functionality which totally relies on the species or strain of bacteria. Some probiotics were reported to increase the activity of Natural Killer (NK) cells [44-46] serve as a first line of defense because they carried out cytotoxic actions without previous antigen sensitization. Probiotic-containing yogurt, milk, or sausages significantly increased the activity of the Natural Killer (NK) cells and their percentage in the blood peripheral of human volunteers [47-51]. L. casei DNl 14001 consumption increases the oxidative burst capacity of Natural Killer (NK) cells and monocytes which results in a positive effect in modulating innate immune defense [52]. Studies showed that the administration of Lactobacillus casei strain Shirota (LcS) not only enhances innate immunity by stimulating the splenic NK cells activity stated by Matsuzaki and Chin [53]. LcS stimulates the production of TH l cytokines which represses the IgE antibodies production against ovalbumin in experimental mice [16,54,55].
Diarrhoea
Diarrhea was the most common adverse effect of both long-term and short-term antibiotic treatment, particularly throughout several antibiotic regimens. Gill and Guamer, 2004; Saad, et al. 2013 [56,57] reported that the cases of antibiotic-associated diarrhea in infants and adults reduced when the patients were co-administration on probiotics. To restore the balance of the intestinal microflora, administration of an exogenous probiotic preparation was required shown in Table 2. Various clinical trial studies had been administrated to check the potency of probiotics in preventing acute diarrhoeal conditions [58-61]. Clostridium difficile a pathogenic strain caused about 10% to 20% of antibiotic-associated diarrhea. Hickson, et al. 2007 [62] reported that the consumption of probiotic drinks containing L. casei, L. bulgaricus and S. thermophilus as starter cultures reduced the chances of antibiotic-associated diarrhea. Probiotics had been used to prevent C. difficile-associated diarrhea in elderly patients who were on antibiotic therapy [62-64].
| Table 2: Probiotics used treatment durations and dosages. | ||
| Probiotic(s) Used (Genus and Strain) | Duration of Treatment | Dosage |
| Lactobacillus acidophilus | 10 days | 5.1 × 108 CFU |
| Lactobacillus bulgaricus | 10 days | 5.1 × 108 CFU |
| Lactobacillus rhamnosus GG | 7 - 10 days | 2 × 1010 CFU |
| Streptococcus thermophilus | 12 days | 109 CFU |
| Bifidobacterium animalis lactis | 11 days | 109 CFU |
| Lactobacillus casei | 17 days | 1 × 105 CFU |
| Bifidobacterium longum | 12 - 15 days | 1× 106 CFU |
Claeson and Merson, 1990 [65] reported that in indus-trialized countries rotavirus was the most common cause of acute diarrhea among infants. Rotavirus differentiated invades and replicates in absorptive epithelial cells of the small intestine which leads to the disruption of the intestinal mucosa and results in loss of micro-villi, a decrease in the villus/crypt ratio, and also an increase in permeability of the intestine [66-68]. Clinical trials reveal that acute diarrhea conditions were reduced with the intake of probiotics mainly L. reuteri, L. GG, L. casei, and S. Boulardii [56]. The duration of acute diarrhoeal sickness in infants was treated by probiotic therapy for approximately one day. Szajewska, et al. [69] reported that L. GG reduced the period of diarrhea which was mainly induced by rotavirus. LGG also reduced the persistence of diarrhea (lasting greater or equal to seven days) and reduced the duration of hospitalization as compared to a placebo [57]. The diarrhoeal disease was common among travelers and traveler’s diarrhea affects approximately 20% - 50% of travelers replacement of antibacterial drugs with Lactobacilli is considered to be a safe alternative [64,70]. Probiotics by competing with pathogenic viruses or bacteria prevent the binding to epithelial cells [71] or by the production of bacteriocins [64,72] help in preventing microbes that causes diarrhea and against H. pylori [73].
Inflammatory bowel disease
Inflammatory bowel disease IBD, which encompasses ulcerative colitis, pouchitis and Crohn’s disease, is a chronic and recurrent inflammation that typically affects the colon or small intestine [74]. The mucosa and submucosa of the colon were the only parts of ulcerative colitis (UC) where an inflammatory response occurred, and these areas had distinct boundaries. The entire gastrointestinal system was said to be affected by Crohn’s Disease (CD) and the inflammation penetrated the intestinal wall from the mucosa to the outer coat (serosa). While diarrhea, stomach discomfort and weight loss were the prominent symptoms of Crohn’s disease, diarrhea was the main symptom of ulcerative colitis and was frequently accompanied by rectal hemorrhage. Patients with inflammatory bowel disease had higher concentrations of a particular type of bacterium, called Bacteroides, adhered to epithelial cells than did persons without the condition [75]. When compared with healthy people the microbiota with irritable bowel syndrome was shown to be less stable [76-78].
With the restoration of altered intestinal microbiota, probiotics are crucial in the treatment of IBS. According to Hamilton-Miller, treating IBD using L. salivahus UCC118, L. rhamnosus GG, S. cerevisiae (boulardii) and E. coli (Nissle) was successful [79]. Numerous studies reported the potential value of probiotic therapy and show how the combination of strains is crucial for rehabilitation [80-82]. It had also been recommended that individuals with mild cases of ulcerative colitis consume fermented milk with B. breve, B. bifidum, and L. acidophilus [82]. The probiotic VSL#3 combinations were very successful in keeping chronic pouchitis in remission [80,84]. Linskens, et al. (2001) found showed in people with predisposed genetic makeup, the lumenal product from the local flower contributed to the development of mucosal inflammatory reactions. By assessing the impact of regulatory T cells on effector T cell subsets, it was possible to determine that the intestinal mucosa was in a state of regulated inflammation. The effector T cell’s activities prevail and cause pathological inflammation when this regulation is compromised. Probiotics like Lactobacilli and Bifidobacteria, which appear to have anti-inflammatory properties, have been used as an alternative to traditional therapy in the treatment of inflammatory bowel disease [71].
- Arora T, Singh S, Sharma RK. Probiotics: Interaction with gut microbiome and antiobesity potential. Nutrition. 2013 Apr;29(4):591-6. doi: 10.1016/j.nut.2012.07.017. Epub 2013 Jan 1. PMID: 23287068.
- Feizabadi F, Sharifan A, Tajabadi N. Isolation and identification of lactic acid bacteria from stored Apis mellifera honey. Journal of Apicultural Research. 2020; 1–6. doi:10.1080/00218839.2020.1765490.
- Harish K, Varghese T. Probiotics in humans-evidence based review. Calicut. Med. J. 2006; 4(4):e3.
- Bisht N, Garg AP. Role of Gut Microbiota in Human Health. Res. J. Biotech. 2021; 16(1).
- Curk MC, Hubert JC, Bringel F. Lactobacillus paraplantarum sp. now., a new species related to Lactobacillus plantarum. Int J Syst Bacteriol. 1996 Apr;46(2):595-8. doi: 10.1099/00207713-46-2-595. PMID: 15551474.
- Hertzler SR, Clancy SM. Kefir improves lactose digestion and tolerance in adults with lactose maldigestion. J Am Diet Assoc. 2003 May;103(5):582-7. doi: 10.1053/jada.2003.50111. PMID: 12728216.
- Montalto M, Gallo A, Gasbarrini A, Landolfi R. NSAID enteropathy: could probiotics prevent it? J Gastroenterol. 2013 Jun;48(6):689-97. doi: 10.1007/s00535-012-0648-2. Epub 2012 Aug 9. PMID: 22875474.
- Gallo A, Passaro G, Gasbarrini A, Landolfi R, Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J Gastroenterol. 2016 Aug 28;22(32):7186-202. doi: 10.3748/wjg.v22.i32.7186. PMID: 27621567; PMCID: PMC4997632.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014 Aug;11(8):506-14. doi: 10.1038/nrgastro.2014.66. Epub 2014 Jun 10. PMID: 24912386.
- Shermak MA, Saavedra JM, Jackson TL, Huang SS, Bayless TM, Perman JA. Effect of yogurt on symptoms and kinetics of hydrogen production in lactose-malabsorbing children. Am J Clin Nutr. 1995 Nov;62(5):1003-6. doi: 10.1093/ajcn/62.5.1003. PMID: 7572723.
- Saavedra JM. Clinical applications of probiotic agents. Am J Clin Nutr. 2001 Jun;73(6):1147S-1151S. doi: 10.1093/ajcn/73.6.1147S. PMID: 11393193.
- Şanlier N, Gökcen BB, Sezgin AC. Health benefits of fermented foods. Crit Rev Food Sci Nutr. 2019;59(3):506-527. doi: 10.1080/10408398.2017.1383355. Epub 2017 Oct 20. PMID: 28945458.
- Vasiljevic T, Shah NP. Probiotics-From Metchnikoff to bioactives. International Dairy Journal. 2008; 18(7):714–728. doi:10.1016/j.idairyj.2008.03.004
- Kratz M, Cullen P, Wahrburg U. The impact of dietary mono- and poly-unsaturated fatty acids on risk factors for atherosclerosis in humans. Eur J Lipid Sci Technol. 2002; 104:300–311.
- Lardizabal JA, Deedwania P. Lipid-lowering therapy with statins for the primary and secondary prevention of cardiovascular disease. Cardiol Clin. 2011 Feb;29(1):87-103. doi: 10.1016/j.ccl.2010.10.002. PMID: 21257102.
- Agim-Ezenwaka OA, Onuh EF, Ndulaka JC, Onwusiribe UD, Chukwu MN. Organoleptic attributes of legume-yogurt samples fermented by lactic acid bacteria. Noble International Journal of Agriculture and Food Technology. 2019; 1(2):75-85.
- Shaper AG, Jones KW, Jones M, Kyobe J. Serum lipids in three nomadic tribes of Northern Kenya. Am J Clin Nutr. 1963 Sep;13:135-46. doi: 10.1093/ajcn/13.3.135. PMID: 14061585.
- Mann GV. Studies of a surfactant and cholesteremia in the Maasai. Am J Clin Nutr. 1974 May;27(5):464-9. doi: 10.1093/ajcn/27.5.464. PMID: 4596028.
- Harrison VC, Peat G. Serum cholesterol and bowel flora in the newborn. Am J Clin Nutr. 1975 Dec;28(12):1351-5. doi: 10.1093/ajcn/28.12.1351. PMID: 45573.
- Agerbaek M, Gerdes LU, Richelsen B. Hypocholesterolaemic effect of a new fermented milk product in healthy middle-aged men. Eur J Clin Nutr. 1995 May;49(5):346-52. PMID: 7664720.
- Sarkar S. Recent innovations in cultured milk products for infants. Nutrition & Food Science. 2003; 33(6):268- 272. https://doi.org/10.1108/00346650310507091
- Liong MT, Shah NP. Effects of a Lactobacillus casei synbiotic on serum lipoprotein, intestinal microflora, and organic acids in rats. J Dairy Sci. 2006 May;89(5):1390-9. doi: 10.3168/jds.S0022-0302(06)72207-X. PMID: 16606710.
- Yu Z, Zhang X, Li S, Li C, Li D, Yang Z. Evaluation of probiotic properties of Lactobacillus plantarum strains isolated from Chinese sauerkraut. World J Microbiol Biotechnol. 2013 Mar;29(3):489-98. doi: 10.1007/s11274-012-1202-3. Epub 2012 Nov 2. PMID: 23117677.
- Liong MT, Shah NP. Acid and bile tolerance and cholesterol removal ability of Lactobacilli strains. J Dairy Sci. 2005 Jan;88(1):55-66. doi: 10.3168/jds.S0022-0302(05)72662-X. PMID: 15591367.
- Liong MT, Shah NP. Bile salt deconjugation ability, bile salt hydrolase activity and cholesterol co-precipitation ability of Lactobacilli strains. International Dairy Journal. 2005b; 15(4):391–398. doi:10.1016/j.idairyj.2004.08.007.
- Correa CES, Pereira MN, Oliveira Liveira SG and Ramos MH. Performance of holstein cows fed sugarcane or corn silage of different grain textures. Scientia Agricola. 2003; 60:621–629.
- Mahrous H, Mohamed A, El-Mongy MA, El-Batal AI, Hamza HA. Study bacteriocin production and optimization using new isolates of Lactobacillus spp. isolated from some dairy products under different culture conditions. Food Nutr. Sci. 2013; 4:342–356.
- Bisht N, Garg AP. Isolation, characterization and probiotic value of lactic acid bacteria from milk and milk products. Biotechtoday. 2019; 9:54.
- Kumar R, Grover S, Batish VK. Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague-Dawley rats. Br J Nutr. 2011 Feb;105(4):561-73. doi: 10.1017/S0007114510003740. Epub 2010 Oct 6. PMID: 20923582.
- Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006 Jun;100(6):1171-85. doi: 10.1111/j.1365-2672.2006.02963.x. PMID: 16696665.
- Perdigon G, Alvarez S. Probiotics and the immune state. In: Fu¨ller, R. (Coord.), Chapman & Hall, London. 1992.
- Ouwehand AC, Salminen S, Tölkkö S, Roberts P, Ovaska J, Salminen E. Resected human colonic tissue: new model for characterizing adhesion of lactic acid bacteria. Clin Diagn Lab Immunol. 2002 Jan;9(1):184-6. doi: 10.1128/cdli.9.1.184-186.2002. PMID: 11777852; PMCID: PMC119867.
- Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. 1992 Aug;32(2):141-4. doi: 10.1203/00006450-199208000-00002. PMID: 1324462.
- Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol. 1994 Nov;10(1):55-63. doi: 10.1111/j.1574-695X.1994.tb00011.x. Erratum in: FEMS Immunol Med Microbiol 1995 Dec;12(3-4):273. Erratum in: FEMS Immunol Med Microbiol 1995 Sep;12(1):83. PMID: 7874079.
- Nikawa H, Makihira S, Fukushima H, Nishimura H, Ozaki Y, Ishida K, Darmawan S, Hamada T, Hara K, Matsumoto A, Takemoto T, Aimi R. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int J Food Microbiol. 2004 Sep 1;95(2):219-23. doi: 10.1016/j.ijfoodmicro.2004.03.006. PMID: 15282133.
- Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 2001 Feb;22(2):78-83. doi: 10.1016/s1471-4906(00)01811-1. PMID: 11286707.
- Kinoshita H, Wakahara N, Watanabe M, Kawasaki T, Matsuo H, Kawai Y, Kitazawa H, Ohnuma S, Miura K, Horii A, Saito T. Cell surface glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of Lactobacillus plantarum LA 318 recognizes human A and B blood group antigens. Res Microbiol. 2008 Nov-Dec;159(9-10):685-91. doi: 10.1016/j.resmic.2008.07.005. Epub 2008 Aug 26. PMID: 18790050.
- Schiffrin EJ, Brassart D, Servin AL, Rochat F, Donnet-Hughes A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection. Am J Clin Nutr. 1997 Aug;66(2):515S-520S. doi: 10.1093/ajcn/66.2.515S. PMID: 9250141.
- Schiffrin EJ, Blum S. Interactions between the microbiota and the intestinal mucosa. Eur J Clin Nutr. 2002 Aug;56 Suppl 3:S60-4. doi: 10.1038/sj.ejcn.1601489. PMID: 12142966.
- Mishra V, Goswami P, Dabur R, Prasad DN. Antibiotic susceptibility and in vivo studies of selected probiotic strains of Lactobacillus casei. Int J Prob Preb. 2008; 3(1):15–20.
- Dhewa T, Pant S, Mishra V. Development of freeze dried synbiotic formulation using a probiotic strain of Lactobacillus plantarum. J Food Sci Technol. 2014 Jan;51(1):83-9. doi: 10.1007/s13197-011-0457-2. Epub 2011 Jul 16. PMID: 24426051; PMCID: PMC3857416.
- Marlina E, Hirani B, Mercadante V, Shephard M, Kishida S, Sebepos-Rogers G, Smith A. P028 The anti-inflammatory effects of a poly-probiotic on the oral mucosa. Journal of Crohn’s and Colitis. 2020; 145-146. https://doi.org/10.1093/ecco-jcc/jjz203.157.
- de Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr. 2005 Oct;44(7):406-13. doi: 10.1007/s00394-004-0541-8. Epub 2004 Dec 1. PMID: 15578195.
- Takeda K, Suzuki T, Shimada SI, Shida K, Nanno M, Okumura K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin Exp Immunol. 2006 Oct;146(1):109-15. doi: 10.1111/j.1365-2249.2006.03165.x. PMID: 16968405; PMCID: PMC1809741.
- Takeda K, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the human NK-cell activity. J Nutr. 2007 Mar;137(3 Suppl 2):791S-3S. doi: 10.1093/jn/137.3.791S. Erratum in: J Nutr. 2018 Jan 1;148(1):160. PMID: 17311976.
- Makino S, Sato A, Goto A, Nakamura M, Ogawa M, Chiba Y, Hemmi J, Kano H, Takeda K, Okumura K, Asami Y. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J Dairy Sci. 2016 Feb;99(2):915-923. doi: 10.3168/jds.2015-10376. Epub 2015 Dec 10. PMID: 26686726.
- Spanhaak S, Havenaar R, Schaafsma G. The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Eur J Clin Nutr. 1998 Dec;52(12):899-907. doi: 10.1038/sj.ejcn.1600663. PMID: 9881885.
- Jahreis G, Vogelsang H, Kiessling G, Schubert R, Bunte C, Hammes WP. Influence of probiotic sausage (Lactobacillus paracasei) on blood lipids and immunological parameters of healthy volunteers. Food Research Internationa. 2002; 35:133-138.
- Shu Q, Gill HS. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20) against Escherichia coli O157:H7 infection in mice. FEMS Immunol Med Microbiol. 2002 Sep 6;34(1):59-64. doi: 10.1111/j.1574-695X.2002.tb00603.x. PMID: 12208607.
- Martino ME, Bayjanov JR, Caffrey BE, Wels M, Joncour P, Hughes S, Gillet B, Kleerebezem M, van Hijum SA, Leulier F. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environ Microbiol. 2016 Dec;18(12):4974-4989. doi: 10.1111/1462-2920.13455. Epub 2016 Aug 4. PMID: 27422487.
- Begum R, Sarker AK, Islam A, Alam K, Pramanik K. Isolation and Characterization of Lactic acid Bacteria from Indigenous Dairy Product and Preparation of Starter Culture by Freeze-drying. Bioresearch Communication. 2017; 3(1).
- Parra MD, Martínez de Morentin BE, Cobo JM, Mateos A, Martínez JA. Daily ingestion of fermented milk containing Lactobacillus casei DN114001 improves innate-defense capacity in healthy middle-aged people. J Physiol Biochem. 2004 Jun;60(2):85-91. doi: 10.1007/BF03168444. PMID: 15457926.
- Matsuzaki T, Chin J. Modulating immune responses with probiotic bacteria. Immunol Cell Biol. 2000 Feb;78(1):67-73. doi: 10.1046/j.1440-1711.2000.00887.x. PMID: 10651931.
- Coda R, Cassone A, Rizzello CG, Nionelli L, Cardinali G, Gobbetti M. Antifungal activity of Wickerhamomyces anomalus and Lactobacillus plantarum during sourdough fermentation: identification of novel compounds and long-term effect during storage of wheat bread. Appl Environ Microbiol. 2011 May;77(10):3484-92. doi: 10.1128/AEM.02669-10. Epub 2011 Mar 25. PMID: 21441340; PMCID: PMC3126437.
- Dongarrà ML, Rizzello V, Muccio L, Fries W, Cascio A, Bonaccorsi I, Ferlazzo G. Mucosal immunology and probiotics. Curr Allergy Asthma Rep. 2013 Feb;13(1):19-26. doi: 10.1007/s11882-012-0313-0. PMID: 23054627.
- Gill HS, Guarner F. Probiotics and human health: a clinical perspective. Postgrad Med J. 2004 Sep;80(947):516-26. doi: 10.1136/pgmj.2003.008664. PMID: 15356352; PMCID: PMC1743098.
- Saad N, Delattre C, Urdaci M, Schmitter JM, Bressollier P. An overview of the last advances in probiotic and prebiotic field. LWT - Food Science and Technology. 2013; 50(1):1-16. doi:10.1016/j.lwt.2012.05.014.
- Basu S, Chatterjee M, Ganguly S, Chandra PK. Efficacy of Lactobacillus rhamnosus GG in acute watery diarrhoea of Indian children: a randomised controlled trial. J Paediatr Child Health. 2007 Dec;43(12):837-42. doi: 10.1111/j.1440-1754.2007.01201.x. Epub 2007 Sep 4. PMID: 17803667.
- Fang SB, Lee HC, Hu JJ, Hou SY, Liu HL, Fang HW. Dose-dependent effect of Lactobacillus rhamnosus on quantitative reduction of faecal rotavirus shedding in children. J Trop Pediatr. 2009 Oct;55(5):297-301. doi: 10.1093/tropej/fmp001. Epub 2009 Feb 8. PMID: 19203988.
- Grandy G, Medina M, Soria R, Terán CG, Araya M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis. 2010 Aug 25;10:253. doi: 10.1186/1471-2334-10-253. PMID: 20735858; PMCID: PMC2940902.
- Amato M, Di Spirito F, D'Ambrosio F, Boccia G, Moccia G, De Caro F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms. 2022 Nov 18;10(11):2289. doi: 10.3390/microorganisms10112289. PMID: 36422359; PMCID: PMC9694231.
- Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, Bulpitt CJ. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007 Jul 14;335(7610):80. doi: 10.1136/bmj.39231.599815.55. Epub 2007 Jun 29. PMID: 17604300; PMCID: PMC1914504.
- Thompson J, Gregory S, Plummer S, Shields RJ, Rowley AF. An in vitro and in vivo assessment of the potential of Vibrio spp. as probiotics for the Pacific white shrimp, Litopenaeus vannamei. J Appl Microbiol. 2010 Oct;109(4):1177-87. doi: 10.1111/j.1365-2672.2010.04743.x. Epub 2010 Aug 19. PMID: 20477892.
- Nishioka H, Mizuno T, Iwahashi H, Horie M. Changes in lactic acid bacteria and components of Awa-bancha by anaerobic fermentation. Biosci Biotechnol Biochem. 2020 Sep;84(9):1921-1935. doi: 10.1080/09168451.2020.1771677. Epub 2020 May 28. PMID: 32463340.
- Claeson M, Merson MH. Global progress in the control of diarrheal diseases. Pediatr Infect Dis J. 1990 May;9(5):345-55. doi: 10.1097/00006454-199005000-00008. PMID: 2191271.
- Salim R, Ben-Shlomo I, Colodner R, Keness Y, Shalev E. Bacterial colonization of the uterine cervix and success rate in assisted reproduction: results of a prospective survey. Hum Reprod. 2002 Feb;17(2):337-40. doi: 10.1093/humrep/17.2.337. PMID: 11821274.
- Gilboa Y, Bar-Hava I, Fisch B, Ashkenazi J, Voliovitch I, Borkowski T, Orvieto R. Does intravaginal probiotic supplementation increase the pregnancy rate in IVF-embryo transfer cycles? Reprod Biomed Online. 2005 Jul;11(1):71-5. doi: 10.1016/s1472-6483(10)61301-6. PMID: 16102292.
- Gomand F, Borges F, Salim D, Burgain J, Guerin J, Gaiani C. High-throughput screening approach to evaluate the adhesive properties of bacteria to milk biomolecules. Food Hydrocoll. 2018; 84:537–544. doi: 10.1016/j.foodhyd.2018.06.038.
- Szajewska H. Probiotics and prebiotics in pediatrics: where are we now? Turk J Pediatr. 2007 Jul-Sep;49(3):231-44. PMID: 17990574.
- de Roos NM, Katan MB. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am J Clin Nutr. 2000 Feb;71(2):405-11. doi: 10.1093/ajcn/71.2.405. PMID: 10648252.
- Kelly P, Maguire PB, Bennett M, Fitzgerald DJ, Edwards RJ, Thiede B, Treumann A, Collins JK, O'Sullivan GC, Shanahan F, Dunne C. Correlation of probiotic Lactobacillus salivarius growth phase with its cell wall-associated proteome. FEMS Microbiol Lett. 2005 Nov 1;252(1):153-9. doi: 10.1016/j.femsle.2005.08.051. Epub 2005 Sep 19. PMID: 16214296.
- Miraglia del Giudice M, De Luca MG. The role of probiotics in the clinical management of food allergy and atopic dermatitis. J Clin Gastroenterol. 2004 Jul;38(6 Suppl):S84-5. doi: 10.1097/01.mcg.0000133293.18576.d2. PMID: 15220666.
- Bisht N, Garg AP. Helicobacter pylori Gastric Infection: Pathogenesis and Clinical Management. Intech Open. 2022. DOI: http://dx.doi.org/10.5772/intechopen.106783
- Weersma RK, Stokkers PC, van Bodegraven AA, van Hogezand RA, Verspaget HW, de Jong DJ, van der Woude CJ, Oldenburg B, Linskens RK, Festen EA, van der Steege G, Hommes DW, Crusius JB, Wijmenga C, Nolte IM, Dijkstra G; Dutch Initiative on Crohn and Colitis (ICC). Molecular prediction of disease risk and severity in a large Dutch Crohn's disease cohort. Gut. 2009 Mar;58(3):388-95. doi: 10.1136/gut.2007.144865. Epub 2008 Sep 29. PMID: 18824555.
- Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, Hale LP, Lochs H. Adherent biofilms in bacterial vaginosis. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1013-23. doi: 10.1097/01.AOG.0000183594.45524.d2. PMID: 16260520.
- Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005 Sep 1;22(5):387-94. doi: 10.1111/j.1365-2036.2005.02579.x. PMID: 16128676.
- Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008 Jan 1;27(1):48-57. doi: 10.1111/j.1365-2036.2007.03542.x. Epub 2007 Oct 5. PMID: 17919270.
- Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005 Feb;100(2):373-82. doi: 10.1111/j.1572-0241.2005.40312.x. PMID: 15667495.
- Seksik P, Dray X, Sokol H, Marteau P. Is there any place for alimentary probiotics, prebiotics or synbiotics, for patients with inflammatory bowel disease? Mol Nutr Food Res. 2008 Aug;52(8):906-12. doi: 10.1002/mnfr.200700147. PMID: 18384087.
- Lammers KM, Helwig U, Swennen E, Rizzello F, Venturi A, Caramelli E, Kamm MA, Brigidi P, Gionchetti P, Campieri M. Effect of probiotic strains on interleukin 8 production by HT29/19A cells. Am J Gastroenterol. 2002 May;97(5):1182-6. doi: 10.1111/j.1572-0241.2002.05693.x. PMID: 12014725.
- Gupta P, Andrew H, Kirschner BS, Guandalini S. Is lactobacillus GG helpful in children with Crohn's disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr. 2000 Oct;31(4):453-7. doi: 10.1097/00005176-200010000-00024. PMID: 11045848.
- Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P, Rizzello F, Campieri M, Brigidi P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol. 2008 Jul 31;125(3):286-92. doi: 10.1016/j.ijfoodmicro.2008.04.012. Epub 2008 Apr 30. PMID: 18524406.
- Chiellini E, Barghini A, Cinelli P and Ilieva VI. Overview of environmentally compatible polymeric materials for food packaging. Environmentally Compatible Food Packaging. 2008; 371-395. doi:10.1533/9781845694784.3.371.
- Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004 Jan;53(1):108-14. doi: 10.1136/gut.53.1.108. PMID: 14684584; PMCID: PMC1773918.