More Information
Submitted: February 17, 2023 | Approved: March 29, 2023 | Published: March 30, 2023
How to cite this article: Essuman VA, Benjamin A, Essuman A, Akpalu J, Sackey AH, et al. Quality of life in Ghanaian children and adolescents with type 1 diabetes mellitus compared with non diabetic controls and caregivers’ report. J Adv Pediatr Child Health. 2023; 6: 014-021.
DOI: 10.29328/journal.japch.1001055
Copyright License: © 2023 Essuman VA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Caregiver; Children and adolescents; Type 1 diabetes mellitus; Ghanaian; Quality of life
Quality of life in Ghanaian children and adolescents with type 1 diabetes mellitus compared with non diabetic controls and caregivers’ report
Essuman VA1, Abaidoo Benjamin1* , Essuman A2, Akpalu J3, Sackey AH4, Barnes NA5 and Amoaku WM6
, Essuman A2, Akpalu J3, Sackey AH4, Barnes NA5 and Amoaku WM6
1Ophthalmology Unit, Department of Surgery, University of Ghana Medical School, Accra, Ghana
2Department of Internal Medicine and Therapeutics, University of Health and Allied Sciences, Ho, Ghana
3Department of Medicine and Therapeutics, University of Ghana Medical School, Accra, Ghana
4Department of Child Health, University of Ghana Medical School, Accra, Ghana
5Santa Rosa Community Health, Vista Clinic 3569 Round Barn Circle, Santa Rosa, CA 95403, USA
6Academic Ophthalmology, Mental Health & Clinical Neurosciences, University of Nottingham Medical School, University Hospital, QMC Nottingham, UK
*Address for Correspondence: AbaidooBenjamin, Ophthalmology Unit, Department of Surgery, The University of Ghana Medical School, University of Accra, Ghana, Email: [email protected]
Background: Measurement of health-related quality of life (HRQOL) in children and adolescents with type 1 diabetes mellitus (T1DM) is as important as metabolic control in the management and prevention of diabetes-related complications.
Aim: To describe the self-reported HRQOL outcomes in Ghanaian children and adolescents with T1DM compared with healthy controls and perceived HRQOL by caregivers.
Setting: Out-patient clinics of the Departments of Child Health, Medicine and Therapeutics, Family Medicine, and Ophthalmology, the National Diabetes Management and Research Centre (all at the Korle Bu Teaching Hospital), and the Cape Coast Teaching Hospital (CCTH).
Methods: Socio-demographic and clinical characteristics of study participants were documented. Participants completed the PedsQL™ 4.0 Generic Core Scales. Data analysis was done with SPSS Version 25.0. An unpaired t-test was used in comparing the HRQOL scores between children and adolescents with T1DM and controls, and parental proxy reports.
Results: Fifty children and adolescents with T1DM, 50 parents/caregivers, and 80 healthy non-diabetic controls took part in this study. There was no significant difference in mean score between the patients and the caregivers for overall HRQOL (p = 0.270). Patients reported significantly worse overall HRQOL than their controls (p = 0.001). Males with diabetes reported better HRQOL than females (p = 0.007).
Conclusion: Children and adolescents with T1DM and their parents/caregivers reported lower HRQOL scores compared to healthy controls. Males reported better HRQOL than females.
Potential implications: HRQOL should be routinely assessed together with proxy reports from parents to identify those who might benefit from further attention including referral to a psychologist.
Diabetes Mellitus (DM) is a cluster of metabolic diseases with associated complications affecting the eyes, kidneys, and other organs [1-3]. DM is known to affect all ages [4] and is a global epidemic. A global estimate in 2022 shows that a total of 8.75 million people have T1D, with one-fifth (1.9 million) of these persons living in low-income and lower-middle-income nations [5]. Seventeen percent (1.52 million) of the 8.75 million people with T1D are children and adolescents younger than 20 years [5]. In Ghana, out of the estimated 10,925 individuals with T1D, about 2,761 are known to be children and adolescents under 20 years [5]. Developmental changes including pubertal hormonal and psychosocial change, and the demands of DM, in general, may complicate glycaemic control in children and adolescents with T1DM [6]. Modern diabetes care for children and adolescents has evolved from main drug treatment to a holistic approach involving the achievement of optimal glycaemic control, normal psychological development, and maximum health-related quality of life (HRQOL) [7].
DM in children and adolescents is usually seen as a family disease rather than a disease of the affected individual. This is because of the integral role of family relationships and parental support associated with its management [8]. T1DM complications inflict a burden on both the affected child and caregivers, requiring the adoption of multiple lifestyle and psychosocial changes in behavior [9]. Depression, anxiety, stress-related mental disorders, and disruption in academic activities have been reported to be more common among children and adolescents with T1DM, compared to their healthy colleagues [7,10,11].
The effects of DM on several domains of HRQOL such as school functioning, physical and emotional well-being, and social interactions in children and adolescents and their carers have been described [7]. Measurement of HRQOL in children and adolescents with DM offers an opportunity to detect persons with low HRQOL and to apply appropriate early interventional measures [7]. Studies have indicated that by improving the quality of life (QOL) and well-being of children and adolescents with DM, the occurrence of complications associated with DM can be prevented [7,12].
A few studies have reported low parental perception of QOL compared with self-reports of the QOL of children and adolescents with DM [13,14]. However, to the best of our knowledge, there is limited information about self-reported QOL in Ghanaian children and adolescents with T1DM compared with their healthy colleagues and with the perceived QOL by their caregivers. In this study, we describe the self-reported HRQOL in Ghanaian children and adolescents with T1DM compared with healthy controls and with their caregivers’ perceived QOL reports.
Study design
This was a cross-sectional study involving children and adolescents with T1DM and age-matched non-diabetic controls.
Setting
Study participants were recruited from the out-patient clinics of the Departments of Child Health, Medicine and Therapeutics, Family Medicine, Ophthalmology, and the National Diabetes Management and Research Centre (all at the Korle Bu Teaching Hospital, Accra), and the Cape Coast Teaching Hospital (CCTH) from March 2016 to September 2019. Healthy controls were from selected educational facilities in Accra.
Study population and sampling strategy
The study population included children and adolescents with T1DM aged 4-19 years and their caregivers conveniently sampled from the outpatient clinics above, and age-matched non-diabetic controls conveniently sampled from selected educational facilities in Accra.
Data collection
Each participant gave consent or assent to be part of the study. Participating institutions gave permission for the study to be conducted. Subjects with acute disease during the study period were excluded. Healthy controls were children and adolescents without diabetes or other acute or chronic diseases. A detailed study protocol has been previously published elsewhere [15].
Demographic characteristics (age, sex, level of education) and clinical information on patients including past medical history, features of any systemic complications, laboratory investigations, blood pressure measurements, and treatment modalities used were documented on a data collection form.
T1DM was confirmed by the presence of glutamic acid decarboxylase (GAD) antibodies, insulinopenia, or low levels of C-peptide [16-18]. Duration of DM was categorized as long duration (> 24 months after diagnosis) and short duration (≤ 24 months after diagnosis) [19]. Measurements of participants’ height in meters (m) and weight in kilograms (kg) were taken and their body mass index (BMI) (kg/m2) was determined. Age and sex-standardized BMI z-scores were computed and classified using the World Health Organization (WHO) growth standards for BMI in children and adolescents aged 5-19 years [20].
Glycated hemoglobin (HbA1c) was measured using the Tri-Stat Boronate Affinity System (Trinity Biotech, Ireland). Patients with HbA1c < 7.0% or ≥ 7.0% were said to have good or poor glycemic control respectively [21].
The PedsQL™ 4.0 Generic Core Scale developed by Varni and colleagues [22,23] was used in assessing the self-reported HRQOL in study participants. The PedsQL™ 4.0 Generic Core Scale is a 5-point Likert scale ranging from 0 (Never) to 4 (Almost Always) without the weighing of items on the scale. Permission was sought from the Mapi Research Trust (Lyon, France) for the use of the HRQOL tool in this study.
Data analysis
Data analysis was done using SPSS for Windows, version 25.0 (SPSS Inc., Chicago, Ill., USA). HRQOL scores of the participants with T1DM were compared with those of healthy controls and the proxy report from their parents/caregivers using the PedsQL™ 4.0 Generic Core Scales. Items on the tool were reversed, scored, and linearly transformed to a 0-100 scale as follows: 0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0. Scale scores were not computed and more than 50% of the items in the scale were missing. The mean of the completed items in the scale was computed by summing the items over the total number of items answered. The Psychosocial Health Summary Score was obtained by the summation of the items over the number of items answered in the Emotional, Social, and School Functioning Scales. The Physical Health Summary Score is the score of the physical functioning scale. The total score was finally obtained by summing all the items over the number of items answered on all the Scales. Overall QoL scores were categorized as “good/better” (81% - 100 %), and “poor” (below 81%).
Descriptive statistics were reported as mean ± standard deviation, median (interquartile range), percentages, and frequency distribution respectively. The Chi-square test or Fisher’s exact test was used in examining the association between demographic characteristics and HRQOL. Carl Pearson’s correlation was used in examining the relationship between the overall HRQOL score and the mean HbA1c. An unpaired t-test (independent t - test) was used in comparing the HRQOL scores between the patients with DM and their healthy controls and with proxy reports from parents/caregivers. P - values < 0.05, were considered statistically significant.
Ethical considerations
Ethical approval was obtained from the Ethical and Protocol Review Committee (EPRC) of the University of Ghana Medical School with Protocol Identification Number: MS-Et/M.12-P4.5/2013-2014).
Demographic characteristics of study participants
A total of 50 children and adolescents with T1DM, 50 parents/caregivers, and 80 healthy controls were recruited. Table 1 presents the demographic characteristics of the children and adolescents with T1DM and controls.
For the children and adolescents with T1DM, the mean (± SD) and median (IQR) ages were 14.6 ± 2.8 years and 15.0 (13.0-17.0) years; minimum and maximum ages were 5 and 19 years respectively. Most (38, 76.0%) were aged 13-19 years. The majority (38, 76.0%) were females and were mostly at the Basic School (29, 58.0%) level of education. Most (31, 62.0%) had normal weight with mean and median BMI; of 20.0 ± 4.1 kg/m2 and 20.2 (16.8-22.1) kg/m2, minimum and maximum BMI; 11.1 kg/m2 and 29.3 kg/m2 respectively.
The mean and median ages for the controls were 13.6 ± 3.2 years and 14.0 (11.3-16.0) years; minimum and maximum ages were 5 years and 18 years respectively. Most (54, 67.5%) were aged 13-19 years. The majority (56, 70.0%) were females and most (52, 65.0%) were at the Basic School level of education. The controls had a mean and median BMI of 20.5 ± 2.3 kg/m2 and 19.9 (18.8-21.8) kg/m2, the minimum and maximum BMI were 16.8 kg/m2 and 31.0 kg/m2 (Table 1).
| Table 1: Demographic characteristics of children and adolescents with diabetes and control group. | ||
| Characteristic | Children and adolescents with T1DM (N = 50) N (%) | Control group (N = 80) N (%) |
| Age group: | ||
| 5-7 | 1 (2.0) | 3 (3.8) |
| 8-12 | 11 (22.0) | 23 (28.7) |
| 13-19 | 38 (76.0) | 54 (67.5) |
| BMI categories (z-score): | ||
| Severe thinness (< -3) | 3 (6.0) | 6 (7.5) |
| Thinness (< -2 to -3) | 3 (6.0) | 7 (8.8) |
| Normal (-2 to 1) Overweight (> 1to 2) Obese (> 2) |
38 (76.0) 5 (10.0) 1 (2.0) |
55 (68.8) 9 (11.2) 3 (3.7) |
| Sex: | ||
| Male | 12 (24.0) | 24 (30.0) |
| Female | 38 (76.0) | 56 (70.0) |
| Education: | ||
| Basic school | 29 (58.0) | 52 (65.0) |
| Senior High School | 20 (40.0) | 28 (35.0) |
| Tertiary | 1 (2.0) | 0 (0) |
The majority (45, 90.0%) of the parent/caregiver respondents were females. The mean age of the parents was 51.9 ± 4.6 years. The minimum age was 45.0 years and the maximum was 62.0 years. Most (46, 92.0%) of the parents/caregivers were employed, and majority (29, 58.0%) had Senior High School education.
Diabetes-specific data of children and adolescents with T1DM
The mean and median ages at diagnosis of DM were 10.4 ± 3.4 years and 11.0 (9.0-13.3) years. The mean and median duration of DM was 21.0 ± 26.3 months and 9.5 (4.0-30.0) months. The maximum duration of DM was 98 months and the minimum was 1 month. The mean and median Hb1Ac were 10.4 ± 2.9% and 9.9 (8.4%-12.4%). The duration of T1DM was short (≤ 24 months) in most (37, 74.0%) participants. All (50, 100.0%) of the children and adolescents with T1DM in this study were on premixed insulin twice daily. Only 5 (10.0%) of the children and adolescents with T1DM had good glycaemic control and 45 (90.0%) had poor glycaemic control (Table 2).
| Table 2: Diabetes-specific data of children and adolescents with type1 diabetes mellitus. | ||||
| Characteristics | Number (n) |
Percentage (%) |
Mean ± standard deviation | Median (IQR) |
| Duration of diabetes (mths): | ||||
| Short (≤ 24mths) | 37 | 74.0 | - | - |
| Long (> 24mths) | 13 | 26.0 | - | - |
| Total | 50 | 100.0 | 21.0 ± 26.3 | 9.5 (4.0-30.0) |
| Age at diagnosis (years) | - | - | 10.4 ± 3.4 | 11.0 (9.0-13.3) |
| Type of treatment: | ||||
| Insulin | 50 | 100.0 | - | - |
| Type of insulin: | ||||
| Premixed | 50 | 100.0 | - | - |
| Parental Hx DM | 18 | 36.0 | - | - |
| Glycaemic control: | ||||
| Good | 5 | 10.0 | - | - |
| Poor | 45 | 90.0 | - | - |
| HBA1C | - | - | 10.4 ± 2.9 | 9.9 (8.4-12.4) |
| IQR: Interquartile Range; mths: months; DM: Diabetes Mellitus; Hx: History; HbA1c: HaemoglobinA1c | ||||
HRQOL scores for children and adolescents with T1DM and their caregivers report
In an independent t-test analysis, there was no significant difference in mean score between the patients and the caregivers’ proxy report in all the dimensions (physical functioning, emotional functioning, social functioning, school functioning, psychosocial health status) and the overall HRQOL (p > 0.05) (Table 3).
| Table 3: HRQOL scores for children and adolescents with T1DM and caregivers’ proxy report. | ||||
| HRQOL Dimension Patients' Caregivers | Mean values | Difference | p - value | |
| Mean ± SD | Mean ± SD | |||
| Physical functioning | 77.7 ± 22.3 | 68.9 ± 22.0 | 8.8 | 0.068 |
| Emotional functioning | 67.3 ± 22.4 | 71.0 ± 17.8 | 3.7 | 0.397 |
| Social functioning | 83.3 ± 19.0 | 78.9 ± 22.1 | 4.4 | 0.31 |
| School functioning | 68.8 ± 23.2 | 61.8 ± 18.9 | 7.1 | 0.124 |
| Psychosocial health | 73.1 ± 18.9 | 70.5 ± 15.2 | 2.6 | 0.483 |
| Overall | 74.4 ± 19.3 | 70.1 ± 15.8 | 4.2 | 0.27 |
| T1DM: Type 1 Diabetes Mellitus; HRQOL: Health-Related Quality of Life; SD: Standard Deviation. | ||||
HRQOL scores for children and adolescents with T1DM and controls
Children and adolescents with T1DM recorded a poor overall HRQOL score (74.4 ± 19.3) compared to their healthy colleagues (84.9 ± 13.8), (p = 0.001). All the dimensions of HRQOL recorded a poor HRQOL among the patients compared to their healthy colleagues with a significant difference in mean HRQOL score between the patients and their healthy colleagues (p < 0.05) except in the social functioning dimension which did not record a significant difference (p = 0.345) (Table 4).
| Table 4: HRQOL scores for children and adolescents with T1DM and controls. | ||||
| HRQOL Dimension | Mean values | Difference | p - value | |
| Patients | Controls | |||
| Mean ± SD | Mean ± SD | |||
| Physical functioning | 77.7 ± 22.3 | 86.5 ± 15.4 | 8.8 | 0.001 |
| Emotional functioning | 67.3 ± 22.4 | 81.6 ± 17.2 | 14.3 | 0.001 |
| Social functioning | 83.3 ± 19.0 | 83.7 ± 15.2 | 2.7 | 0.345 |
| School functioning | 68.8 ± 23.2 | 87.8 ± 19.7 | 19 | 0.001 |
| Psychosocial health | 73.1 ± 18.9 | 84.3 ± 14.0 | 11.2 | 0.001 |
| Overall | 74.4 ± 19.3 | 84.9 ± 13.8 | 10.5 | 0.001 |
| T1DM: Type 1 Diabetes Mellitus; HRQOL: Health-Related Quality of Life; SD: Standard Deviation. | ||||
Association between HRQOL and glycaemic control in children and adolescents with T1DM
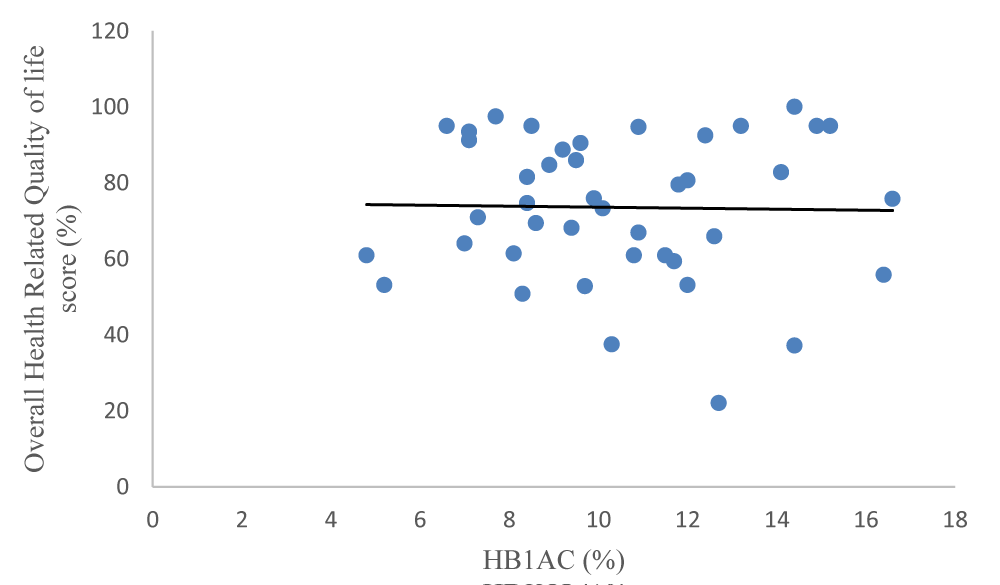
In terms of all the dimensions in the HRQOL general score scale, there was no significant difference between children and adolescents who had good glycaemic control and those with poor glycaemic control (p > 0.05). The overall HRQOL did not show any significant difference in mean scores between those with good glycaemic control versus poor control (p = 0.460) (Table 5). Carl Pearson’s correlation between the overall HRQOL score and the mean HbA1c (r2 = -0.020, p = 0.900) was not statistically significant as shown in Figure 1.
Figure 1: Overall HRQOL score and HbA1c (%)
| Table 5: Association between HRQOL scores and glycaemic control of children and adolescents with T1DM. | ||||
| HRQOL Dimension | Mean values | Difference | p - value | |
| Good control | Poor control | |||
| Mean ± SD | Mean ± SD | |||
| Physical functioning | 82.8 ± 20.0 | 77.2 ± 22.7 | 5.6 | 0.635 |
| Emotional functioning | 68.0 ± 13.0 | 67.2 ± 23.3 | 0.8 | 0.942 |
| Social functioning | 78.0 ± 21.7 | 83.9 ± 18.9 | 5.9 | 0.518 |
| School functioning | 73.0 ± 26.4 | 68.3 ± 23.1 | 4.7 | 0.674 |
| Psychosocial health | 73.0 ± 19.9 | 73.1 ± 19.0 | 0.1 | 0.987 |
| Overall | 76.0 ± 22.2 | 74.2 ± 19.3 | 1.8 | 0.861 |
| T1DM: Type 1 Diabetes Mellitus; HRQOL: Health-Related Quality of Life; SD: Standard Deviation. | ||||
Association between demographic and clinical characteristics, and overall HRQOL of children and adolescents with T1DM
Sex was the only variable that was associated with overall HRQOL (p - value = 0.020). Males reported better QoL than females (p = 0.007). Age, BMI, and level of education of patients were not associated with overall HRQOL (p - values > 0.05); and lower Hb1Ac level was not associated with better HRQOL. There was no significant difference between males and females in terms of mean Hb1Ac; 9.7 ± 2.2 vs. 10.6 ± 2.9, p = 0.366. (Table 6).
| Table 6: Association between demographic and clinical characteristics and overall HRQOL of children and adolescents with T1DM. | |||
| Characteristic | Overall HRQOL | P - value | |
| Better | Poor | ||
| Age groups | |||
| 5-7 | 0 ((0) | 1 (2.0) | 0.512 |
| 8-12 | 6 (12.0) | 5 (10.0) | |
| 13-19 | 16 (32.0) | 22 (44.0) | |
| Sex | |||
| Females | 13 (26.0) | 25 (50.0) | 0.020* |
| Males | 9 (18.0) | 3 (6.0) | |
| Education | |||
| Basic | 14 (28.0) | 15 (30.0) | 0.344 |
| SHS | 7 (14.0) | 13 (26.0) | |
| Tertiary | 1 (2.0) | 0 (0) | |
| Duration of diabetes (mths) | |||
| Short (≤ 24 mths) | 18 (36.0) | 20 (40.0) | 0.512 |
| Long (>24 mths) | 4 (8.0) | 8 (16.0) | |
| BMI categories (z-score): | 0.065 | ||
| Severe thinness (< -3) | 2 (4.0) | 1 (2.0) | |
| Thinness (<-2 to -3) | 2 (4.0) | 1 (2.0) | |
| Normal (-2 to 1) | 17 (34.0) | 20 (40.0) | |
| Overweight (>1to 2) | 0 (0.0) | 6 (12.0) | |
| Obese (> 2) | 1 (2.0) | 0 (0.0) | |
| Lower Hb1Ac | |||
| Yes | 4 (8.0) | 35 (10.0) | 1 |
| No | 18 (36.0) | 23 (46.0) | |
| HRQOL: Health-Related Quality of Life; BMI: Body Mass Index. Education: SHS: Senior High School; HBA1C: HaemoglobinA1C. Males reported better HRQoL than females (p = 0.007). There was no significant difference between males and females in terms of mean Hb1Ac; 9.7 ± 2.2 vs. 10.6 ± 2.9, p = 0.366. |
|||
Our results of a lower score for overall HRQOL in children and adolescents with T1DM compared to their healthy colleagues is consistent with those from similar studies among children and adolescents with T1DM from Europe and Asia [24–27]. This finding is possibly linked with the psychological trauma and burden associated with the diagnosis of DM and its demands of management, requiring the adoption of multiple lifestyle and psychosocial changes in behavior in order to achieve a desirable level of glycemic control. This usually brings disruption in family patterns and routines, and drives the need to adopt and adjust to prescribed patterns of diet, administration of insulin, and regular monitoring of blood glucose levels [28]. Children and adolescents with T1DM have poor glycaemic control compared to the general pediatric population and are more likely to be non-adherent to treatment regimens which could impact negatively their HRQOL. For example, the acute effects of high as well as low blood glucose levels can serve as a barrier and limit social interactions, and their psychological well-being and interfere with their daily activities such as exercise and school performance [12,29]. Poor HRQOL among children and adolescents with T1DM has also been partly attributed to having single-parent families or families with disadvantaged socioeconomic status [30,31]. However, this issue was not examined in this study.
Studies have shown that at least 15% of children and adolescents with T1DM experience mental stress during the treatment of DM [9,32]. Van Tilburg, et al. [33] reported an increased rate of depressive symptoms among children with T1DM compared to their healthy colleagues. Other studies have also shown associations between stigmatization and ‘diabetic distress’ in children and adolescents with diabetes, often characterized by suboptimal glycaemic control, low self-efficacy, and reduced self-care [34-36]. The low HRQOL scores within the emotional and school functioning dimensions reported for children and adolescents with T1DM compared to the controls in our study is consistent with those from other studies [24,27]. A lack of autonomy in the management of the condition and the challenges of preoccupation associated with chronic complications among children and adolescents with T1DM may account for the low level of emotional HRQOL [13,14]. Healthcare providers, especially primary care physicians should look out for symptoms of depression in these children and manage them with proven interventions. As a result of the need to frequently see their physicians, these children may need to be absent from school, which may affect their academic activities and performance [10,11]. More flexible school schedules accommodating diabetes management may help such children and adolescents in adapting to their condition and socializing with minimal intervention. Another intervention is having direct access to a psychologist with an interest in diabetes, ideally being present in the diabetes clinic; to assess the family at diagnosis and identify those who require more intensive support. Other useful strategies may include increasing teacher knowledge to provide more individualized support and enhancing the quality of communication between parents and school staff [37].
Parental psychological responses to having a child with T1DM are therefore crucial in guiding clinical practice and future research. The reported prevalence of parental psychological distress across several studies ranged from 10% to 74%, with an average of 33.5% of parents reporting distress at diagnosis and 19% reporting distress within 1 to 4 years after diagnosis of a child with T1DM [38].
Previous studies found parents/caregivers reporting lower scores than their teens [7,24,27]. Possible reasons were the burden of T1DM on parents, differences in parental and adolescent’s cognitive development, risk evaluation, and behavior regulation by adolescents [39]. Our findings were similar although this difference was not statistically significant.
The association of the male gender with a better HRQOL in our study supports evidence from other studies [24,26,40]. Girls with T1DM have been reported to have more diabetes-related worries and may take responsibility for their disease earlier because they tend to enter puberty and mature earlier than boys [41]. Also, sex differences in physical and hormonal changes that occur during puberty lead to girls requiring higher insulin doses compared to boys. [42]. A study in the Middle East has shown that females with DM have double the prevalence rates of depression compared to males [43]. Huang, et al. [44] and De Wit, et al. [45] however, did not find any association between gender and HRQOL.
Other studies have reported an association between the duration of T1DM and HRQOL [24,41]. Our study did not establish any association between the duration of diabetes (shorter or longer) and HRQOL (p = 0.507). This could be attributed to the fact that most of the participants in the study had a duration of less than 2yrs.
Previous studies have reported conflicting findings regarding glycaemic control and HRQOL [19,40]. Laffel, et al. did not find any association of HbA1c with HRQOL [19], similar to the present study. Various studies have demonstrated the association of higher HRQOL scores with better glycaemic control [24,26,40,46-48]. Other studies have also shown that poorer HRQOL is associated with higher HbA1c levels and greater depressive symptoms in the pediatric population with T1DM [49,50]. Among children and adolescents with T1DM, failure to establish and achieve glycaemic control may be quite frustrating leading to a lower scoring of HRQOL by the children. Psychosocial factors such as better parental support have been found to be associated with better glycaemic control among the pediatric population with T1DM [9,28].
This study is one of the few studies that used the PedsQL Generic Core Scale in examining the HRQOL among children and adolescents with T1DM in the Sub-Saharan African region. The outcome of this study supports recommendations to use reports from children together with proxy reports from parents in determining a child’s HRQOL [51,52]. Another strength of this study is the multidisciplinary collaboration involving pediatric diabetologists, ophthalmologists, physicians, biostatisticians, and public health practitioners.
The limitations of this study include the use of a general measure of the quality of life rather than a diabetes-specific measure, which could limit the ability to identify different dimensions of diabetes management that negatively impact HRQOL. Additionally, the sample size may be too small to generalize the findings of this study to the whole population. A larger population study in the future, where both general and diabetes-specific HRQOL are evaluated across several socio-demographic and clinical characteristics would be useful. A baseline measurement of HbA1c may not adequately determine the glycaemic control of the participants.
Children and adolescents with T1DM and their parents/caregivers reported lower HRQOL scores compared to healthy controls. Glycaemic control measured by HbA1c value was not significantly associated with HRQOL. Males reported better HRQOL than females among children and adolescents with DM. In the management of children and adolescents with T1DM, HRQOL should be routinely assessed in order to identify those who might benefit from further attention including referral to a psychologist.
Mapi Research Trust granted permission for use of the study tool (PedsQL™, Copyright © 1998 JW Varni, Ph.D. All rights reserved). PedsQL™contact information and permission to use: Mapi Research Trust, Lyon, France.Internet: https://eprovide.mapi-trust.org and www.pedsql.org.
We are grateful to the executives and members of Diabetes Youth Care- Ghana (DYC-Ghana) and all the children and adolescents with diabetes enrolled in the study and their caregivers for their support. Special thanks to the staff of the Eye Department at the Cape-Coast Teaching Hospital, and the National Diabetes Management and Research Centre, at the Korle-Bu Teaching Hospital (KBTH), for their cooperation during the conduct of this study. Our sincere thanks also go to Drs. Tagoe NN, Hayfron-Benjamin CF, Asare G, Amoah AGB, Ndanu TA, Ofori-Adjei IDB, and Appiah-Thompson BL.
Funding
Support from the University of Ghana Research Fund.
Data availability
Data for this study is available upon a reasonable request to the corresponding author.
Disclaimer
Views expressed in this article are opinions of the authors and not an official position of the institution or funder.
- AMBOSS. Diabetes mellitus. 2019; https://www.amboss.com/us/knowledge/Diabetes_mellitus. Accessed 10-05-21.
- Donaghue KC, Craig ME, Chan AK, Fairchild JM, Cusumano JM, Verge CF, Crock PA, Hing SJ, Howard NJ, Silink M. Prevalence of diabetes complications 6 years after diagnosis in an incident cohort of childhood diabetes. Diabet Med. 2005 Jun;22(6):711-8. doi: 10.1111/j.1464-5491.2005.01527.x. PMID: 15910621.
- Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K. Microvascular and macrovascular complications associated with diabetes in children and adolescents. Pediatr Diabetes. 2009 Sep;10 Suppl 12:195-203. doi: 10.1111/j.1399-5448.2009.00576.x. PMID: 19754630.
- https://diabetesatlas.org/atlas/tenth-edition/ Accessed on 7th January, 2023.
- Ogle GD, Wang F, Gregory GA, Maniam J. Type 1 diabetes estimates in children and adults – 2022.
- Dumont M, Provost MA. Resilience in adolescents: protective role of social support, coping strategies, self-esteem and social activities on experience of stress and depression. J Youth Adolesc. 1999; 3:343–363.
- Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003 Mar;26(3):631-7. doi: 10.2337/diacare.26.3.631. PMID: 12610013.
- Moore SM, Hackworth NJ, Hamilton VE, Northam EP, Cameron FJ. Adolescents with type 1 diabetes: parental perceptions of child health and family functioning and their relationship to adolescent metabolic control. Health Qual Life Outcomes. 2013 Mar 22;11:50. doi: 10.1186/1477-7525-11-50. PMID: 23521786; PMCID: PMC3614451.
- Delamater AM, de Wit M, McDarby V, Malik J, Acerini CL; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. Psychological care of children and adolescents with type 1 diabetes. Pediatr Diabetes. 2014 Sep;15 Suppl 20:232-44. doi: 10.1111/pedi.12191. PMID: 25182317.
- Wolfsdorf JI, Allgrove J, Craig ME, Edge J, Glaser N, Jain V, Lee WW, Mungai LN, Rosenbloom AL, Sperling MA, Hanas R; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014 Sep;15 Suppl 20:154-79. doi: 10.1111/pedi.12165. Epub 2014 Jul 12. PMID: 25041509.
- International Hypoglycaemia Study Group. Glucose Concentrations of Less Than 3.0 mmol/L (54 mg/dL) Should Be Reported in Clinical Trials: A Joint Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017 Jan;40(1):155-157. doi: 10.2337/dc16-2215. Epub 2016 Nov 21. PMID: 27872155.
- de Wit M, Delemarre-van de Waal HA, Bokma JA, Haasnoot K, Houdijk MC, Gemke RJ, Snoek FJ. Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: a randomized controlled trial. Diabetes Care. 2008 Aug;31(8):1521-6. doi: 10.2337/dc08-0394. Epub 2008 May 28. PMID: 18509204; PMCID: PMC2494630.
- Laffel LM, Connell A, Vangsness L, Goebel-Fabbri A, Mansfield A, Anderson BJ. General quality of life in youth with type 1 diabetes: relationship to patient management and diabetes-specific family conflict. Diabetes Care. 2003 Nov;26(11):3067-73. doi: 10.2337/diacare.26.11.3067. PMID: 14578241.
- Grey M, Boland EA, Yu C, Sullivan-Bolyai S, Tamborlane WV. Personal and family factors associated with quality of life in adolescents with diabetes. Diabetes Care. 1998 Jun;21(6):909-14. doi: 10.2337/diacare.21.6.909. PMID: 9614606.
- Essuman VA, Tagoe NN, Akpalu J, Essuman A, Sackey AH, Hayfron-Benjamin CF, Asare G, Abaidoo B, Amoah A, Ndanu T, Ofori-Adjei I, Barnes NA, Appiah-Thompson BL, Amoaku WM. Morbidity and Complications of Diabetes Mellitus in Children and Adolescents in Ghana: Protocol for a Longitudinal Study. JMIR Res Protoc. 2021 Jan 6;10(1):e21440. doi: 10.2196/21440. PMID: 33404517; PMCID: PMC7817364.
- Katz LE, Jawad AF, Ganesh J, Abraham M, Murphy K, Lipman TH. Fasting c-peptide and insulin-like growth factor-binding protein-1 levels help to distinguish childhood type 1 and type 2 diabetes at diagnosis. Pediatr Diabetes. 2007 Apr;8(2):53-9. doi: 10.1111/j.1399-5448.2007.00236.x. PMID: 17448127.
- Amutha A, Datta M, Unnikrishnan R, Anjana RM, Mohan V. Clinical profile and complications of childhood- and adolescent-onset type 2 diabetes seen at a diabetes center in south India. Diabetes Technol Ther. 2012 Jun;14(6):497-504. doi: 10.1089/dia.2011.0283. Epub 2012 May 2. PMID: 22551567.
- Chiang JL, Maahs DM, Garvey KC, Hood KK, Laffel LM, Weinzimer SA, Wolfsdorf JI, Schatz D. Type 1 Diabetes in Children and Adolescents: A Position Statement by the American Diabetes Association. Diabetes Care. 2018 Sep;41(9):2026-2044. doi: 10.2337/dci18-0023. Epub 2018 Aug 9. PMID: 30093549; PMCID: PMC6105320.
- Abdul-Rasoul M, AlOtaibi F, Abdulla A, Rahme Z, AlShawaf F. Quality of life of children and adolescents with type 1 diabetes in Kuwait. Med Princ Pract. 2013;22(4):379-84. doi: 10.1159/000347052. Epub 2013 Feb 15. PMID: 23428425; PMCID: PMC5586761.
- WHO. Growth reference data for BMI-for-age 5-19 years. [Internet]. Geneva: WHO; [updated 2021 May 16; cited 2022 June 10]. https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age.
- American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020 Jan;43(Suppl 1):S163-S182. doi: 10.2337/dc20-S013. PMID: 31862756.
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999 Feb;37(2):126-39. doi: 10.1097/00005650-199902000-00003. PMID: 10024117.
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001 Aug;39(8):800-12. doi: 10.1097/00005650-200108000-00006. PMID: 11468499.
- Samardzic M, Tahirovic H, Popovic N, Popovic-Samardzic M. Health-related quality of life in children and adolescents with type 1 diabetes mellitus from Montenegro: relationship to metabolic control. J Pediatr Endocrinol Metab. 2016 Jun 1;29(6):663-8. doi: 10.1515/jpem-2015-0420. PMID: 27054599.
- Graue M, Wentzel-Larsen T, Bru E, Hanestad BR, Søvik O. The coping styles of adolescents with type 1 diabetes are associated with degree of metabolic control. Diabetes Care. 2004 Jun;27(6):1313-7. doi: 10.2337/diacare.27.6.1313. PMID: 15161781.
- Nansel TR, Weisberg-Benchell J, Wysocki T, Laffel L, Anderson B; Steering Committee of the Family Management of Diabetes Study. Quality of life in children with Type 1 diabetes: a comparison of general and diabetes-specific measures and support for a unitary diabetes quality-of-life construct. Diabet Med. 2008 Nov;25(11):1316-23. doi: 10.1111/j.1464-5491.2008.02574.x. PMID: 19046222; PMCID: PMC2597420.
- Kalyva E, Malakonaki E, Eiser C, Mamoulakis D. Health-related quality of life (HRQoL) of children with type 1 diabetes mellitus (T1DM): self and parental perceptions. Pediatr Diabetes. 2011 Feb;12(1):34-40. doi: 10.1111/j.1399-5448.2010.00653.x. PMID: 20546163.
- Wysocki T, Nansel TR, Holmbeck GN, Chen R, Laffel L, Anderson BJ, Weissberg-Benchell J; Steering Committee of the Family Management of Childhood Diabetes Study. Collaborative involvement of primary and secondary caregivers: associations with youths' diabetes outcomes. J Pediatr Psychol. 2009 Sep;34(8):869-81. doi: 10.1093/jpepsy/jsn136. Epub 2008 Dec 26. PMID: 19112077; PMCID: PMC2729681.
- Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2010 Aug;22(4):405-11. doi: 10.1097/MOP.0b013e32833a46a7. PMID: 20489639; PMCID: PMC3159529.
- Delamater AM, Shaw KH, Applegate EB, Pratt IA, Eidson M, Lancelotta GX, Gonzalez-Mendoza L, Richton S. Risk for metabolic control problems in minority youth with diabetes. Diabetes Care. 1999 May;22(5):700-5. doi: 10.2337/diacare.22.5.700. PMID: 10332669.
- Silverstein J, Cheng P, Ruedy KJ, Kollman C, Beck RW, Klingensmith GJ, Wood JR, Willi S, Bacha F, Lee J, Cengiz E, Redondo MJ, Tamborlane WV; Pediatric Diabetes Consortium. Depressive Symptoms in Youth With Type 1 or Type 2 Diabetes: Results of the Pediatric Diabetes Consortium Screening Assessment of Depression in Diabetes Study. Diabetes Care. 2015 Dec;38(12):2341-3. doi: 10.2337/dc15-0982. Epub 2015 Oct 12. PMID: 26459274.
- Kongkaew C, Jampachaisri K, Chaturongkul CA, Scholfield CN. Depression and adherence to treatment in diabetic children and adolescents: a systematic review and meta-analysis of observational studies. Eur J Pediatr. 2014 Feb;173(2):203-12. doi: 10.1007/s00431-013-2128-y. PMID: 23959326.
- Van Tilburg MA, McCaskill CC, Lane JD, Edwards CL, Bethel A, Feinglos MN, Surwit RS. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med. 2001 Jul-Aug;63(4):551-5. doi: 10.1097/00006842-200107000-00005. PMID: 11485108.
- Liu NF, Brown AS, Folias AE, Younge MF, Guzman SJ, Close KL, Wood R. Stigma in People With Type 1 or Type 2 Diabetes. Clin Diabetes. 2017 Jan;35(1):27-34. doi: 10.2337/cd16-0020. Erratum in: Clin Diabetes. 2017 Oct;35(4):262. Folias AE [added]. PMID: 28144043; PMCID: PMC5241772.
- Stewart SM, Rao U, White P. Depression and diabetes in children and adolescents. Curr Opin Pediatr. 2005 Oct;17(5):626-31. doi: 10.1097/01.mop.0000176441.49743.ea. PMID: 16160538.
- Hagger V, Hendrieckx C, Sturt J, Skinner TC, Speight J. Diabetes Distress Among Adolescents with Type 1 Diabetes: a Systematic Review. Curr Diab Rep. 2016 Jan;16(1):9. doi: 10.1007/s11892-015-0694-2. PMID: 26748793.
- Fried L, Vithiatharan R, Davis E, Jones T, Hancock K, Runions K, Cross D, Payne D, Jones C, Wright A, Pieterse D, Knowles J, Clarke J, Lin A. The school experiences of children and adolescents with type 1 diabetes in Western Australia. Issues in Educational Research. 2018; 28(3): 578.
- Whittemore R, Jaser S, Chao A, Jang M, Grey M. Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. Diabetes Educ. 2012 Jul-Aug;38(4):562-79. doi: 10.1177/0145721712445216. Epub 2012 May 11. PMID: 22581804; PMCID: PMC3401246.
- AlBuhairan F, Harrison A, Kaufman M, Areemit R. Adolescent psychosocial development and evaluation: global perspectives. INTECH Open Access Publisher; 2012.
- Wagner VM, Müller-Godeffroy E, von Sengbusch S, Häger S, Thyen U. Age, metabolic control and type of insulin regime influences health-related quality of life in children and adolescents with type 1 diabetes mellitus. Eur J Pediatr. 2005 Aug;164(8):491-6. doi: 10.1007/s00431-005-1681-4. Epub 2005 May 5. PMID: 15875213.
- Nardi L, Zucchini S, D'Alberton F, Salardi S, Maltoni G, Bisacchi N, Elleri D, Cicognani A. Quality of life, psychological adjustment and metabolic control in youths with type 1 diabetes: a study with self- and parent-report questionnaires. Pediatr Diabetes. 2008 Oct;9(5):496-503. doi: 10.1111/j.1399-5448.2008.00414.x. Epub 2008 May 28. PMID: 18507786.
- International Diabetes Federation HTaSA. The challenge of adolescence; hormonal changes and sensitivity to insulin. 2008; 52. http://www.idf.org/diabetesvoice/articles/the-challengeof-adolescence-hormonal-changes-and-sensitivityto-insulin
- AlBuhairan FS, Tamim H, Al Dubayee M, AlDhukair S, Al Shehri S, Tamimi W, El Bcheraoui C, Magzoub ME, de Vries N, Al Alwan I. Time for an Adolescent Health Surveillance System in Saudi Arabia: Findings From "Jeeluna". J Adolesc Health. 2015 Sep;57(3):263-9. doi: 10.1016/j.jadohealth.2015.06.009. PMID: 26299553.
- Huang GH, Palta M, Allen C, LeCaire T, D'Alessio D; Wisconsin Diabetes Registry. Self-rated health among young people with type 1 diabetes in relation to risk factors in a longitudinal study. Am J Epidemiol. 2004 Feb 15;159(4):364-72. doi: 10.1093/aje/kwh055. PMID: 14769640.
- de Wit M, Delemarre-van de Waal HA, Bokma JA, Haasnoot K, Houdijk MC, Gemke RJ, Snoek FJ. Self-report and parent-report of physical and psychosocial well-being in Dutch adolescents with type 1 diabetes in relation to glycemic control. Health Qual Life Outcomes. 2007 Feb 16;5:10. doi: 10.1186/1477-7525-5-10. PMID: 17306021; PMCID: PMC1802741.
- Nikitina IL, Kelmanson IA. Health-related quality of life in 4-to-6-year-old children with type 1 diabetes mellitus estimated by children and their mothers. Eur J Pediatr. 2022 Feb;181(2):549-560. doi: 10.1007/s00431-021-04239-0. Epub 2021 Aug 23. PMID: 34424400; PMCID: PMC8380516.
- Hesketh KD, Wake MA, Cameron FJ. Health-related quality of life and metabolic control in children with type 1 diabetes: a prospective cohort study. Diabetes Care. 2004 Feb;27(2):415-20. doi: 10.2337/diacare.27.2.415. PMID: 14747222.
- Hoey H, Aanstoot HJ, Chiarelli F, Daneman D, Danne T, Dorchy H, Fitzgerald M, Garandeau P, Greene S, Holl R, Hougaard P, Kaprio E, Kocova M, Lynggaard H, Martul P, Matsuura N, McGee HM, Mortensen HB, Robertson K, Schoenle E, Sovik O, Swift P, Tsou RM, Vanelli M, Aman J. Good metabolic control is associated with better quality of life in 2,101 adolescents with type 1 diabetes. Diabetes Care. 2001 Nov;24(11):1923-8. doi: 10.2337/diacare.24.11.1923. PMID: 11679458.
- Frøisland DH, Graue M, Markestad T, Skrivarhaug T, Wentzel-Larsen T, Dahl-Jørgensen K. Health-related quality of life among Norwegian children and adolescents with type 1 diabetes on intensive insulin treatment: a population-based study. Acta Paediatr. 2013 Sep;102(9):889-95. doi: 10.1111/apa.12312. Epub 2013 Jul 16. PMID: 23738648.
- Lawrence JM, Yi-Frazier JP, Black MH, Anderson A, Hood K, Imperatore G, Klingensmith GJ, Naughton M, Mayer-Davis EJ, Seid M; SEARCH for Diabetes in Youth Study Group. Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. J Pediatr. 2012 Aug;161(2):201-7.e2. doi: 10.1016/j.jpeds.2012.01.016. Epub 2012 Feb 22. PMID: 22361221; PMCID: PMC4503360.
- Yi-Frazier JP, Hilliard ME, Fino NF, Naughton MJ, Liese AD, Hockett CW, Hood KK, Pihoker C, Seid M, Lang W, Lawrence JM. Whose quality of life is it anyway? Discrepancies between youth and parent health-related quality of life ratings in type 1 and type 2 diabetes. Qual Life Res. 2016 May;25(5):1113-21. doi: 10.1007/s11136-015-1158-5. Epub 2015 Oct 14. PMID: 26466834; PMCID: PMC4936832.
- Hilliard ME, Monaghan M, Cogen FR, Streisand R. Parent stress and child behaviour among young children with type 1 diabetes. Child Care Health Dev. 2011 Mar;37(2):224-32. doi: 10.1111/j.1365-2214.2010.01162.x. Epub 2010 Nov 18. PMID: 21083686.