More Information
Submitted: July 18, 2023 | Approved: July 27, 2023 | Published: July 28, 2023
How to cite this article: Balyorugulu GG, Ambrose E, Ngoya PS, Jamnagerwalla JS, Bur IS, et al. Factors Associated with Elevated Transcranial Doppler Ultrasound Velocities in Children With Sickle Cell Anemia in Mwanza, Tanzania. J Adv Pediatr Child Health. 2023; 6: 033-038.
DOI: 10.29328/journal.japch.1001058
Copyright License: © 2023 Balyorugulu GG, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Factors Associated with Elevated Transcranial Doppler Ultrasound Velocities in Children With Sickle Cell Anemia in Mwanza, Tanzania
Georgina George Balyorugulu1*, Emmanuela Ambrose2, Patrick S Ngoya3, Yusuf S Jamnagerwalla2, Iddi S Buri4, Primrose Songoro4 and Protas D Komba4
1Catholic University of Health and Allied Sciences, Mwanza, Tanzania
2Department of Pediatrics and Child Health, Bugando Medical Centre, Mwanza, Tanzania
3Department of Radiology, Bugando Medical Centre, Mwanza, Tanzania
4Bugando Medical Centre, Mwanza, Tanzania
*Address for Correspondence: Georgina George Balyorugulu, Catholic University of Health and Allied Sciences, P. O. Box 1464, Mwanza, Tanzania, Email: [email protected]
Background: Stroke occurs in 11% of patients with SCA before 20 years of age. In Northwestern Tanzania, the prevalence of stroke among children living with SCA under the age 15 years is 16.9%, of which might be attributed to the absence of routine screening for the risk of stroke by using Transcranial Doppler Ultrasound (TCD). Screening with TCD allows preventive measures such as chronic blood transfusion to be done which has led to the reduction of stroke by 92%.
Methods: This was a prospective analytical cross sectional study which enrolled 267 SCA children aged 2 to 16 years attending Bugando Medical Centre Pediatric Sickle Cell Clinic from July 2019 to June 2020. Assessment of factors associated with elevated TCD included a clinical history of stroke in sibling, death in sibling, temperature, oxygen saturation in room air, blood pressure, hemoglobin level and total white blood cell count. TCD was done by accessing transtemporal window and recording the highest time average mean of maximum velocity (TAMMV) of major vessels mainly, middle cerebral artery (MCA) and distal internal carotid artery (dICA).
Results: The median age of enrolled was 6.6 (IQR: 4-9) years. The prevalence of elevated TCD (> 170 cm/s) was found to be 21% (56/267). By multivariate logistic regression, low oxygen saturation in room air, p - value = 0.037, OR 1.08 [95% CI 1.00-1.17] and low hemoglobin level, p - value = 0.001, OR 1.76 [95% CI 1.26-2.45] were statistically significantly associated with elevated TCD among children living with SCA.
Conclusion: The high prevalence of elevated TCD velocity, with low hemoglobin and low oxygen saturation in room air as associated factors under multivariate logistic regression, warrants routine TCD screening for children with SCA aged 2 to 16 years.
Sickle Cell Anemia (SCA) is a disorder constituting a substitution of an amino acid, glutamine with valine, after a single base pair change of thymine for adenine at the 6th codon of the β globin gene in chromosome 11 [1]. This substitution causes the red blood cell to change into a sickle shape, specifically when a child/patient is under hypoxic conditions because of polymerization of the hemoglobin molecules [1]. As one of the major complications, cerebral vascular diseases may manifest into an overt. The development of stroke is linked to the deformity of the red blood cells whereby the lysed red blood cells release arginase and other adhesions molecules that lead to reduced level of nitrous oxide [2,3] this vicious cycle results in poor flow and finally stroke.
Tanzania ranks fourth worldwide among countries affected by sickle cell disease [4]. The high disease burden is mostly seen in the Lake Zone regions of the country [5,6]. Despite being highly affected with SCA and having a prevalence of stroke of 16.9% in SCA (carrying HbSS) children less than fifteen years of age [7], with BMC being a zonal consultant hospital in the Lake Zone offering sickle cell clinic services, still, screening by using TCD is not routinely being practiced. This creates a gap in offering standard stroke prevention care which in turn will not only reduce mortality and morbidity but also reduce cost to the health system and the child’s family.
Prevalence of abnormal TCD screening has been fluctuating in several studies. Meanwhile, in the STOP trial reported a prevalence of abnormal TCD of 6.72% [8] that of a study done in Kenya showed 0% had abnormal TCD with conditional TCD being 3% [9], eliciting the need to fully understand the situation in our setting.
The study specific objectives were to determine the prevalence of elevated TCD velocities and factors associated with elevated TCD velocities in children with SCA.
Study area
This study was done at Bugando Medical Centre (BMC) Outpatient Sickle Cell Clinic, a zonal consultant and teaching hospital with 950 bed capacity. It caters to the Lake Zone (with a geographic area of 11, 4348 km2) which has a population of 16 million from the following regions; Kagera (25, 265 km2), Geita (20, 054 km2), Mwanza (9, 467 km2), Shinyanga (18, 901 km2), Simiyu (18, 901 km2) and Mara (21, 760 km2) [10,11]. The Pediatric outpatient sickle cell disease annually caters to 550 enrolled children living with sickle cell disease [6,12].
Study design
An analytical cross-sectional study was carried out from July 2019 to June 2020.
Study population
The study population included children with sickle cell anemia aged 2 to 16 years attending BMC pediatric outpatient sickle cell clinic. Age range was selected based on report that sickle cell stroke affects children and adolescents aged 2 to 20 years [13].
Eligibility criteria
The inclusion criteria was children aged 2 to 16 years and homozygous SS. Exclusion criteria was children who had received blood less than 2 weeks before the date of enrollment, febrile illness in the past 2 weeks, inpatient, patients who were in hydroxyurea therapy and patients with stroke (as reported and verified by a neurologist in prior clinic visits or during hospitalization).
Sampling procedure
Two hundred and sixty seven Participants were enrolled serially, as they attended the Sickle Cell Clinic at BMC during the designated study period once consent and assent was obtained.
Informed consent was obtained to per take in the study, then evaluation for the inclusion and exclusion criteria was done. For those enrolled, a clinical examination and laboratory checklist form was used to collect information (demographic data, temperature, oxygen saturation, blood pressure, hemoglobin level, total white blood cell count and TCD screening findings).
A structural clinical and laboratory standardized data collection tool was used to collect demographic, clinical, capture laboratory data and maximum TCD velocity for each participant. Demographic data collected entailed age, gender, history of stroke in sibling and death history in a sibling. Clinical data included; temperature, oxygen saturation by a pulse oximeter machine and blood pressure. Then a physical examination was performed followed by a Transcranial Doppler ultrasonography, which recorded the TAMMV (Time Averaged Mean of Maximum Velocity) of the bilateral middle cerebral and internal carotid arteries.
Assessment of elevated TCD velocities
The TCD examination was performed by using a non-imaging TCD ultrasonography machine-VIASYS Healthcare, serial label SN: PVK0254 with a Doppler Box with DWL Doppler system and QL software using a 2MHZ single element handheld non-imaging pulsed transducer, this was placed at the temporal regions (transtemporal) of the child. Measurements of the major arteries of the circle of Willis (MCA and dICA) were read from both sides of the head (left and right) following the STOP protocols for stroke prevention trial in SCA (STOP) [14]. The TCD machine records a non-imaging spectrum whereby the x axis captures the time with the y axis reporting on the velocity as centimeters per second (cm/s). The highest recorded velocity was obtained from TAMMV as a final reading.
TCD has been recommended as tool for assessing risk of first stroke [15]. Even though TCD has shown to have a low predictive value of 40% with a sensitivity of 59% and specificity of 95% [16] similar efficacy rates were reported by Hoppe, et al. [17], it has led to a significantly drop of in the prevalence of stroke in children with SCA after detection of risk of stroke and chronic blood transfusion was initiated [18]. It requires the subject to remain awake and calm during the procedure as previously described; this poses a challenge especially in young children. If a child is restless, sleeping or agitated not only makes it difficulty accessing the transtemporal windows but also can cause elevation of the TAMMV of MCA or dICA [18,19].
In order to reduce this operational challenge, children during examination were placed in a comfortable position, laid supine on a bed allowing them to watch television playing soothing kids’ shows or cartoons while the guardian or parent was present during the examination. Inadequate examinations were documented but excluded from the study. The average screening time with TCD was thirty to forty minutes. The TCD examination was supervised and validated by a qualified radiologist at BMC conversant in TCD ultrasonography.
The TCD examination was done with no cost to the participant. Children with elevated findings were enrolled to ancillary study for further follow up for a period of 3 years including TCD, enrollment to hydroxyurea treatment and laboratory parameters.
Laboratory methods
Blood sample was collected by veno puncture (phlebotomy) and a 4mls sample collected in a purple top EDTA collecting tube. The tube was correctly labeled by the participant ID code, date taken, and name of the collector and it was clearly marked it is for purpose of this study by the letters TCD. Then it was stored in a cold box at room temperature and transported to BMC laboratory after enrollment with results of hemoglobin and total white blood cell count recorded.
Data management and statistical analysis
Data was entered into Microsoft excel database, analyzed by using STATA version 13, prevalence of elevated TCD velocity among SCA children was determined, with factors associated with elevated TCD velocities in SCA children determined by univariate logistic regression followed by multivariate logistic regression for factors with p value < 0.2 on univariate regression with odd ratio, 95% confidence interval and p value < 0.05 and was considered statistically significant.
Ethical considerations
Ethical clearance for the research proposal was granted by the joint BMC/CUHAS ethics and review committee with research clearance number CREC/389/2019. Furthermore, written informed consent was ensued to the parents/guardians of children with SCA attending at BMC clinic after they have clearly understood the objective of the study by using an information sheet and consent form. Assent was sought for children above the age of 7 years, the parents or guardians signed the informed consent form. For illiterate parents/guardians, a fingerprint was used instead of a signature.
Study enrollment
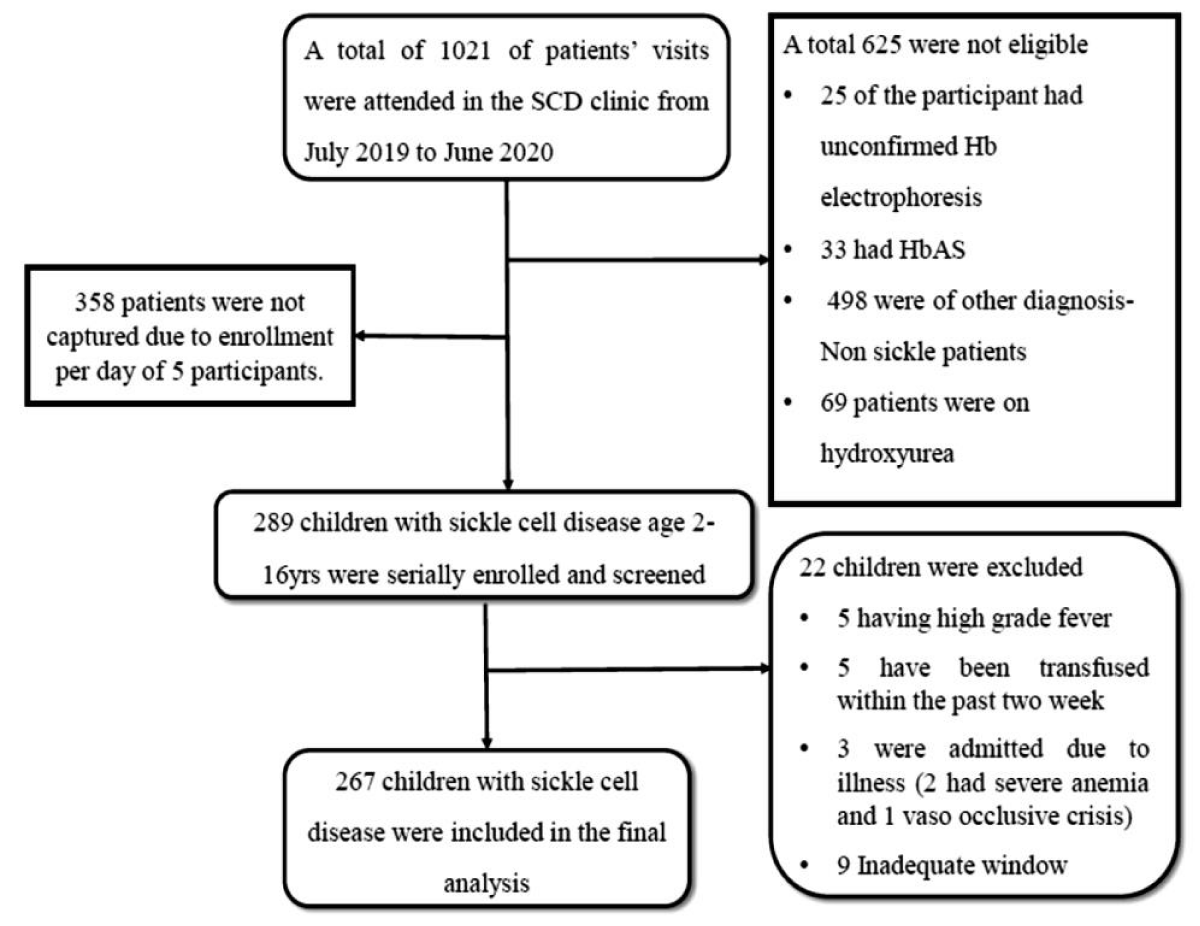
The study was conducted from July 2019 to June 2020. Within the study period a total of 1021 SCD patient visits were attended in the Pediatric Sickle cell clinic. After screening for eligibility, 267 SCA children aged 2 to 16 years were enrolled into the study (Figure 1).
Figure 1: Abdominal US: On the right side of the umbilicus, a 23 mm diameter pathological target sign and approximately a dozen 10 mm lymph nodes are visible. The intussusceptum resolved in a matter of minutes. Radiological opinion: US indicative of intussusception, but no typical clinical signs present.
Baseline characteristics
The median age was 6.6 (interquartile range (IQR): 4 - 9) years with a mean ± SD (96.6 ± 3.5 years). Females were 137 (51.3%). Only one participant had a sibling with sickle cell stroke as reported by the parent (0.4%). (Table 1).
| Table 1: Baseline participants’ characteristics. | |
| Factor | Total of children screened = 267 |
| Age in years, Mean ± SD | 6.62 ± 3.45 |
| Gender Female (%) |
137(51.3) |
| Sibling with stroke Yes (%) No (%) |
1(0.4) 266(99.6) |
| Death history in sibling Yes (%) No (%) |
34(13) 233(87) |
| Temperature(°C), Mean ± SD | 36.48 ± 0.51 |
| Oxygen saturation-SPO2 (%), Mean ± SD | 93.58 ± 3.90 |
| Systolic blood pressure -SBP (mmHg), Mean ± SD | 98.38 ± 9.56 |
| Diastolic blood pressure-DBP (mmHg), Mean ± SD | 60.44 ± 7.26 |
| Hemoglobin (g/dl), Mean ± SD | 7.90 ± 1.34 |
| Total white blood cell count [x109/L], Mean ± SD | 13.81 ± 5.61 |
| Median age in years for elevated TCD (interquartile range) |
6 (4-9.5) |
| Median age in years for abnormal TCD (interquartile range) | 6.5 (5 - 7) |
Outcomes
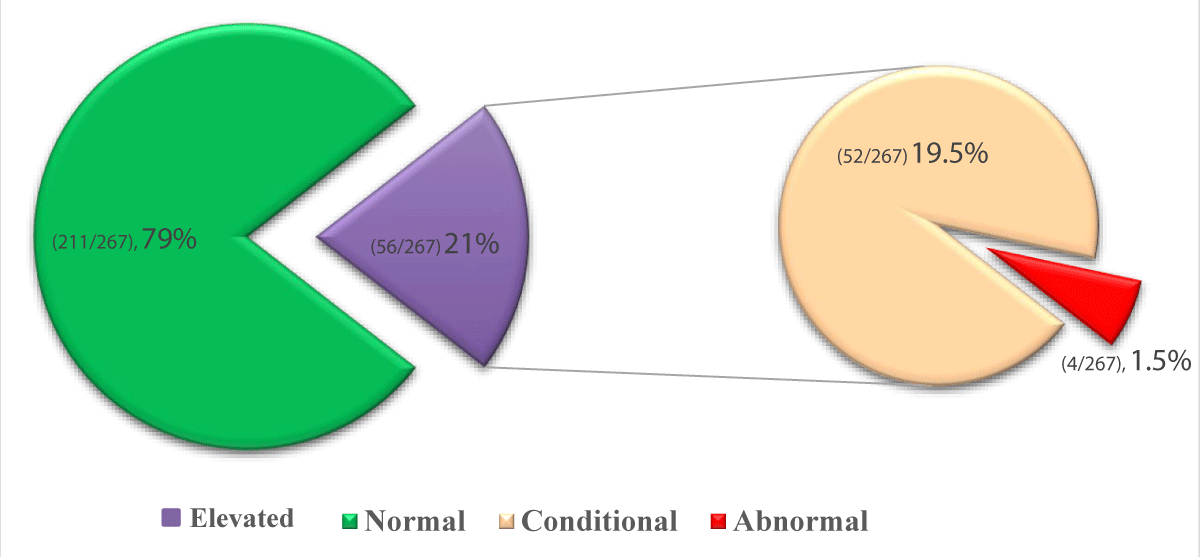
The prevalence of elevated TCD was 21% (56/267). Among those who were screened 211 (79.0%) had normal TCD (≤170 cm/s), 52 (19.5%) had conditional TCD (171 – 200 cm/s) and only 4 (1.5%) had abnormal TCD (≥200) (Figure 2). The overall TAMMV mean ± SD (145.5 ± 25.8 cm/s) and for elevated TCD velocity mean ± SD (181.9 ± 11.2 cm/s).
Figure 2: Prevalence of elevated TCD ultrasonography among SCA children.
In univariate logistic regression, factors associated with elevated TAMMV of TCD ultrasound included high body temperature p = 0.006, OR 2.25 [95% CI 1.26-4.05], low oxygen saturation at room air p = 0.001, OR 0.87 [95% CI 0.82 - 0.95], low hemoglobin level p = <0.001, OR 0.51 [95% CI 0.38 - 0.69] and high white blood cell count p = 0.009, OR 1.08 [95% CI 1.02 - 1.14]. After subjecting independent variables with p - value <0.2 to multivariate regression analysis, low oxygen saturation at room air p - value = 0.037 OR 0.92 [95% CI 0.85 - 0.10] and low hemoglobin level p - value=0.001 OR 0.57 [95% CI 0.41 - 0.79] remained statistically significantly associated with elevated TAMMV of TCD (Table 2).
| Table 2: Factors associated with elevated TCD in SCA children aged 2 to 16 years. | ||||||
| Factor | TCD-TAMMV | Univariate | Multivariate | |||
| Elevated N = 56 |
Normal N = 211 |
OR [95%CI] | p-value | OR [95%CI] | p-value | |
| Age, years (IQR) | 6 (4 - 9.5) | 6 (4 - 9) | 1.00 [0.92 -1.09] | 0.937 | ||
| Temperature, °C ± SD | 36.6 ± 0.6 | 36.4 ( ± 0.5) | 2.25 [1.26 - 4.05] | 0.006 | 1.76 [0.94 - 3.28] | 0.075 |
| SBP*, mmHg ± SD | 99.6 ± 10.6 | 98.1 ± 9.3 | 1.02 [0.99 - 1.05] | 0.294 | ||
| DBP*, mmHg ± SD | 60.0 ± 7.7 | 60.6 ± 7.15 | 0.99 [0.95 - 1.03] | 0.590 | ||
| SPO2* ,% (IQR) | 93 (90 - 95) | 94 (92 - 97) | 0.87 [0.82 - 0.95] | 0.001 | 0.92 [0.85 - 0.10] | 0.037 |
| Hemoglobin, g/dl | 7.2 ± 1.1 | 8.1 ± 1.3 | 0.51 [0.38 - 0.69] | <0.001 | 0.57 [0.41 - 0.79] | 0.001 |
| Total white blood cell count, x 109/L (IQR) | 15.6 (12.9 - 17.3) | 12.5 (10.0 - 16.2) | 1.08 [1.02 - 1.14] | 0.009 | 1.03 [0.97 - 1.10] | 0.353 |
| Gender Female |
30(54%) | 107(51%) | 1.12 [0.62 - 2.02] | 0.703 | ||
| Death history in sibling | 9(16%) | 25(12%) | 1.20[0.64 - 2.26] | 0.577 | ||
| Bolded results are statistically significant at p ≤ 0.05 by Multivariate logistic regression, OR denoted Odds ratio and 95% CI denote 95% confidence interval. | ||||||
Prevalence of elevated TCD
Sickle cell anemia carries a risk of stroke with eleven percent of children and adolescent acquiring stroke before the age of twenty years [20]. The elevated TCD prevalence in this study is higher than that reported in a study done in Kenya, whereby only 3% had elevated TCD velocity [9]. The difference might be attributed to usage of different TCD machines and mortality differences. Children in Kenya have a high mortality rate and therefore die at a very young age thus failed to be captured [21]. This can be further explained as in the Kenya study none of the children were received penicillin prophylaxis or hydroxyurea [9] insinuating premature death due to sepsis or SCA complications while penicillin prophylaxis is offered to under five SCA children at BMC pediatric sickle cell clinic, with hydroxyurea therapy reserved for those with severe complications.
Similar findings have been observed from studies in Mali and Nigeria with the prevalence of elevated TCD 18% [22] and 24% [23] respectively. A subsequent study in Nigeria showed even higher prevalence of 30% [24]. This reflects the burden of the disease in Sub Saharan Africa, with Tanzania’s Lake Zone showing a higher prevalence of sickle cell trait and subsequently HbSS [5] in our country. In addition, the diversity of genetic β cluster haplotypes distribution [21] among the African countries might also have role in predisposition to elevated TCD and the fluctuations in the prevalence among different countries, although this was not assessed and future studies are needed to evaluate genetic predisposition in our setting.
When compared to other countries apart from the sub-Saharan Africa, a higher prevalence of elevated TCD was observed. In Brazil, Leite, et al. and Silva, et al. reported the prevalence of elevated TCD of 11% [25] and 14% [26] respectively. These lower elevated TCD velocity were also observed at baseline by Bernaudin, et al. in France whereby 10% had elevated TCD [27].
The findings seen in this study for elevated TCD velocity are higher than those seen in the Americas and in France. However, with a conversion rate of 34.5% and a median time of conversion of about 1.1 (range 0.03 - 7) years from conditional to abnormal TCD in SCA as reported by Bernaudin, et al. [27], it can be speculated that there is a higher cumulative risk of conversion to abnormal in our setting and therefore a higher risk of stroke, although a longitudinal cohort study is required to verify this claim.
Factors associated with elevated TCD in SCA
This study showed on univariate regression high temperature, low hemoglobin level, high total white blood cell count and low oxygen saturation in room air were significantly associated with elevated TCD, with multivariate regression eliciting only low hemoglobin level and low oxygen saturation in room air to be associated with elevated TCD.
Several studies have reported analogous findings to this study. In a study done in Muhimbili National Hospital Sickle Cell Clinic located in Tanzania Central Zone whereby low hemoglobin correlated with high TCD [28]. In addition, in Kenya a positive relation to high TCD was observed between oxygen saturation ≤95%, fever and TCD of ≥150 cm/s [9]. Meanwhile Nigeria showed increased cerebral flow velocities with low hemoglobin, low hematocrit and arterial oxygen desaturation [24]. Another study reported that overt stroke is associated with low daytime oxygen desaturation levels [29].
In Brazil, low levels of hemoglobin, high leucocyte count (with no infection) were associated with high baseline TCD [26]. Also in France, abnormal TCD was less in patients with high hemoglobin and more in those with high white blood cell count [20]. These findings are similar to those reported in this study.
Furthermore, elaborating on the linkage between low hemoglobin and elevated TCD in SCA, low hemoglobin level, as a marker of ongoing hemolysis can be an indication of potential vascular injury. The pathological injury occurs as a cascade of events which start or precipitated by hemolysis with a release of red blood cell components into the system including free heme, iron, and reactive oxygen species, vasoactive peptides with platelet and white blood cell aggregation and adhesion to the endothelial wall [30-32].
As a result, the bone marrow responds by production of reticulocytes, which have a higher propensity to adhesion to vascular endothelial wall and thus the pathological endothelial injury viscous cycle is propagated [32] until flow is obstructed and stroke ensue. This pathophysiological injury has also been reported to occur in nocturnal deoxygenation [33]. Additionally, oxygen saturation has been reported to be inversely associated with TCD velocity [34] which further support that the deoxygenation state of hemoglobin contributes to the pathophysiological vessel injury in the brain.
Familial predisposition to stroke has been reported in several studies. In this study only 0.4% reported on familial history of stroke as history of a sibling with stroke. This low prevalence was also seen in Brazil whereby it was 1% [25]. Also due to only one history of sibling with stroke, the statistical correlation of history of stroke in sibling and elevated TCD was not ascertained.
Limitations
TCD was done by trained and certified clinicians then validity assessed by an experienced radiologist, this might subject the study to measurement bias of which was minimized by improving accuracy by following the STOP protocol. The absence of neuroimaging studies failed to report on cerebral vasculopathy in elevated TCD and inadequate windows.
Additionally, TCD screening on average took around thirty to forty minutes secondary to ensuring thorough STOP protocol is followed, the vessels velocity is optimized and the participant is calm and not sleeping. This made time restriction a factor for enrolling participants.
The high prevalence of elevated TCD velocity, with low hemoglobin and low oxygen saturation in room air as associated factors warrants implementation of routine TCD screening for children with SCA aged 2 to 16 years.
Recommendations
Routine TCD velocity screening for children aged 2 to 16 years of children living with SCA. In addition, hemoglobin level and oxygen saturation at room air should be incorporated into the screening tools in addition to TCD and also checked in routine clinic visits with rescreening TCD done if abnormal levels seen in order to ameliorate risk of stroke. Lastly, future longitudinal cohort with randomized sampling should be done in our setting for capturing the cause and effect of associated factors of elevated TCD.
Disclaimer
The article is a dissertation submission to the Catholic University of Health and Allied Sciences as a partial fulfillment of the requirements for the award of degree of masters of medicine (pediatrics and child health) of the catholic university of health and allied sciences.
Source of support
The study is a part of American Society of Haematology global award grant and Cincinati Childrens medical hospital received to Paediatric department haematology section through Dr. Emmanuela E Ambrose.
- Ilesanmi OO. Pathological basis of symptoms and crises in sickle cell disorder: implications for counseling and psychotherapy. Hematol Rep. 2010 Jan 26;2(1):e2. doi: 10.4081/hr.2010.e2. Epub 2010 Apr 13. PMID: 22184515; PMCID: PMC3222266.
- Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005 Dec;115(12):3409-17. doi: 10.1172/JCI25040. Epub 2005 Nov 17. PMID: 16294219; PMCID: PMC1283939.
- Switzer JA, Hess DC, Nichols FT, Adams RJ. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurol. 2006 Jun;5(6):501-12. doi: 10.1016/S1474-4422(06)70469-0. PMID: 16713922.
- WHO. Be smart, Know about Sickle Cell Disease! https://afro.who.int/news/be-smart-know-about-sickle-cell-disease.
- Ambrose EE, Makani J, Chami N, Masoza T, Kabyemera R, Peck RN, Kamugisha E, Manjurano A, Kayange N, Smart LR. High birth prevalence of sickle cell disease in Northwestern Tanzania. Pediatr Blood Cancer. 2018 Jan;65(1):10.1002/pbc.26735. doi: 10.1002/pbc.26735. Epub 2017 Aug 2. PMID: 28766840; PMCID: PMC5701733.
- Ambrose EE SL, Charles M, Hernandez AG, Latham T, Hokororo A, Beyanga M, Tebuka E, Howard TA, Ware RE. Geospatial mapping of sickle cell disease in Northwestern Tanzania: The Tanzanian Sickle Surveillance Study (TS3). Blood. 2018 Nov;132 (Suppl 1):3662.
- Saidi H, Smart LR, Kamugisha E, Ambrose EE, Soka D, Peck RN, Makani J. Complications of sickle cell anaemia in children in Northwestern Tanzania. Hematology. 2016 May;21(4):248-256. doi: 10.1080/10245332.2015.1101976. Epub 2016 Feb 17. PMID: 26868490; PMCID: PMC4972452.
- Adams RJ, Nichols FT, Figueroa R, McKie V, Lott T. Transcranial Doppler correlation with cerebral angiography in sickle cell disease. Stroke. 1992 Aug;23(8):1073-7. doi: 10.1161/01.str.23.8.1073. PMID: 1636180.
- Makani J, Kirkham FJ, Komba A, Ajala-Agbo T, Otieno G, Fegan G, Williams TN, Marsh K, Newton CR. Risk factors for high cerebral blood flow velocity and death in Kenyan children with Sickle Cell Anaemia: role of haemoglobin oxygen saturation and febrile illness. Br J Haematol. 2009 May;145(4):529-32. doi: 10.1111/j.1365-2141.2009.07660.x. Epub 2009 Mar 27. PMID: 19344425; PMCID: PMC3001030.
- www.tawananet.or.tz.
- Sub-Divisional Population Projection for year 2016 and 2017 Based on 2012 population and housing census. National Bureau of statistics.
- BMC. https://www.bugandomedicalcentre.go.tz/.
- Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, Wethers DL, Pegelow CH, Gill FM. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998 Jan 1;91(1):288-94. PMID: 9414296.
- Adams RJ, McKie VC, Brambilla D, Carl E, Gallagher D, Nichols FT, Roach S, Abboud M, Berman B, Driscoll C, Files B, Hsu L, Hurlet A, Miller S, Olivieri N, Pegelow C, Scher C, Vichinsky E, Wang W, Woods G, Kutlar A, Wright E, Hagner S, Tighe F, Waclawiw MA, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. 1998 Feb;19(1):110-29. doi: 10.1016/s0197-2456(97)00099-8. PMID: 9492971.
- Alexandrov AV, Sloan MA, Tegeler CH, Newell DN, Lumsden A, Garami Z, Levy CR, Wong LK, Douville C, Kaps M, Tsivgoulis G; American Society of Neuroimaging Practice Guidelines Committee. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. J Neuroimaging. 2012 Jul;22(3):215-24. doi: 10.1111/j.1552-6569.2010.00523.x. Epub 2010 Oct 26. PMID: 20977531.
- Adams RJ, McKie VC, Carl EM, Nichols FT, Perry R, Brock K, McKie K, Figueroa R, Litaker M, Weiner S, Brambilla D. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997 Nov;42(5):699-704. doi: 10.1002/ana.410420505. PMID: 9392568.
- Hoppe C. Defining stroke risk in children with sickle cell anaemia. Br J Haematol. 2005 Mar;128(6):751-66. doi: 10.1111/j.1365-2141.2004.05310.x. PMID: 15755278.
- Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, Abboud M, Gallagher D, Kutlar A, Nichols FT, Bonds DR, Brambilla D. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998 Jul 2;339(1):5-11. doi: 10.1056/NEJM199807023390102. PMID: 9647873.
- Kuboyama T, Hori A, Sato T, Mikami T, Yamaki T, Ueda S. Changes in cerebral blood flow velocity in healthy young men during overnight sleep and while awake. Electroencephalogr Clin Neurophysiol. 1997 Feb;102(2):125-31. doi: 10.1016/s0921-884x(96)95054-7. PMID: 9060863.
- Bernaudin F, Verlhac S, Chevret S, Torres M, Coic L, Arnaud C, Kamdem A, Hau I, Grazia Neonato M, Delacourt C. G6PD deficiency, absence of alpha-thalassemia, and hemolytic rate at baseline are significant independent risk factors for abnormally high cerebral velocities in patients with sickle cell anemia. Blood. 2008 Nov 15;112(10):4314-7. doi: 10.1182/blood-2008-03-143891. Epub 2008 Sep 4. Erratum in: Blood. 2010 Dec 2;116(23):5079. PMID: 18772456.
- Powars D, Hiti A. Sickle cell anemia. Beta s gene cluster haplotypes as genetic markers for severe disease expression. Am J Dis Child. 1993 Nov;147(11):1197-202. doi: 10.1001/archpedi.1993.02160350071011. PMID: 8237915.
- Dorie A, Guindo A, Saro YS, Toure BA, Fane B, Dembele AK, et al. [Screening of cerebral vasculopathy in sickle cell anemia children using transcranial Doppler]. Arch Pediatr. 2015 Mar;22(3):260-6.
- Lagunju I, Sodeinde O, Telfer P. Prevalence of transcranial Doppler abnormalities in Nigerian children with sickle cell disease. Am J Hematol. 2012 May;87(5):544-7. doi: 10.1002/ajh.23152. Epub 2012 Mar 28. PMID: 22460323.
- Lagunju I, Sodeinde O, Brown B, Akinbami F, Adedokun B. Transcranial Doppler ultrasonography in children with sickle cell anemia: Clinical and laboratory correlates for elevated blood flow velocities. J Clin Ultrasound. 2014 Feb;42(2):89-95. doi: 10.1002/jcu.22099. Epub 2013 Oct 26. PMID: 24166013.
- Leite AC, de Oliveira RV, de Moura PG, Silva CM, Lobo C. Abnormal transcranial Döppler ultrasonography in children with sickle cell disease. Rev Bras Hematol Hemoter. 2012;34(4):307-10. doi: 10.5581/1516-8484.20120078. PMID: 23049447; PMCID: PMC3460401.
- Silva CM, Giovani P, Viana MB. High reticulocyte count is an independent risk factor for cerebrovascular disease in children with sickle cell anemia. Pediatr Blood Cancer. 2011 Jan;56(1):116-21. doi: 10.1002/pbc.22680. Epub 2010 Oct 14. PMID: 20949593.
- Bernaudin F, Verlhac S, Arnaud C, Kamdem A, Chevret S, Hau I, Coïc L, Leveillé E, Lemarchand E, Lesprit E, Abadie I, Medejel N, Madhi F, Lemerle S, Biscardi S, Bardakdjian J, Galactéros F, Torres M, Kuentz M, Ferry C, Socié G, Reinert P, Delacourt C. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011 Jan 27;117(4):1130-40; quiz 1436. doi: 10.1182/blood-2010-06-293514. Epub 2010 Nov 10. PMID: 21068435.
- Cox SE, Makani J, Soka D, L'Esperence VS, Kija E, Dominguez-Salas P, Newton CR, Birch AA, Prentice AM, Kirkham FJ. Haptoglobin, alpha-thalassaemia and glucose-6-phosphate dehydrogenase polymorphisms and risk of abnormal transcranial Doppler among patients with sickle cell anaemia in Tanzania. Br J Haematol. 2014 Jun;165(5):699-706. doi: 10.1111/bjh.12791. Epub 2014 Feb 21. PMID: 24666344; PMCID: PMC4154124.
- Quinn CT, Sargent JW. Daytime steady-state haemoglobin desaturation is a risk factor for overt stroke in children with sickle cell anaemia. Br J Haematol. 2008 Feb;140(3):336-9. doi: 10.1111/j.1365-2141.2007.06927.x. Epub 2007 Nov 27. PMID: 18042265; PMCID: PMC2562641.
- Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci U S A. 2014 Sep 30;111(39):E4110-8. doi: 10.1073/pnas.1405023111. Epub 2014 Sep 15. PMID: 25225402; PMCID: PMC4191786.
- Gladwin MT, Ofori-Acquah SF. Erythroid DAMPs drive inflammation in SCD. Blood. 2014 Jun 12;123(24):3689-90. doi: 10.1182/blood-2014-03-563874. PMID: 24926069; PMCID: PMC4055918.
- Platt OS. Preventing stroke in sickle cell anemia. N Engl J Med. 2005 Dec 29;353(26):2743-5. doi: 10.1056/NEJMp058274. PMID: 16382060.
- Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003 Nov 1;362(9394):1450-5. doi: 10.1016/S0140-6736(03)14689-2. PMID: 14602439.
- Quinn CT, Variste J, Dowling MM. Haemoglobin oxygen saturation is a determinant of cerebral artery blood flow velocity in children with sickle cell anaemia. Br J Haematol. 2009 May;145(4):500-5. doi: 10.1111/j.1365-2141.2009.07652.x. Epub 2009 Mar 5. PMID: 19344400; PMCID: PMC2737449.