More Information
Submitted: April 11, 2023 | Approved: May 04, 2024 | Published: May 06, 2024
How to cite this article: Aldin MN, Fisberg RM, Rogero MM, Sarti FM, Damasceno NRT. Case Report: An Elusive Case of Septic Arthritis. J Adv Pediatr Child Health. 2024; 7: 052-061.
DOI: 10.29328/journal.japch.1001068
Copyright License: © 2024 Aldin MN, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: LDL subfractions, HDL subfractions, adolescents, obesity, atherogenic
Obesity and Sex as Determinants of Atherogenic Risk Associated with High-Density (HDL) and Low-Density Lipoprotein (LDL) Subfractions in Adolescents: A Population-based Study Based on Health Survey of Sao Paulo
Marlene N Aldin, Regina M Fisberg, Marcelo M Rogero, Flavia M Sarti and Nágila RT Damasceno*
Department of Nutrition, Faculty of Public Health, University of Sao Paulo (FSP-USP), Sao Paulo, Brazil
*Address for Correspondence: Nágila Raquel Teixeira Damasceno, Department of Nutrition, School of Public Health, University of Sao Paulo; Av. Dr. Arnaldo, 715; 01246-904, Sao Paulo, SP, Brazil, Email: [email protected]
Background: Worldwide, obesity in adolescents is an epidemiological concern. Overweight and obesity are associated with comorbidities in adult life, such as increased risk of hypertension and other non-communicable diseases. This study investigated possible differences between traditional lipid markers and Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) subfractions in a population-wide representative sample of Brazilian adolescents.
Methods: The individuals included in the study comprise a subsample of the 2015 Health Survey of São Paulo (ISA-Capital 2015). LDL and HDL particle sizes were determined by the Lipoprint® System (Quantimetrix Corporation).
Results: 272 Brazilian adolescents with a mean age of 15.2 ± 2.2 years, of which 47.8% (n = 130) are boys. Analysis of LDL subfractions showed an increase in LDL 2 content in girls (5.7% vs. 5.1%; p = 0.047). This result was associated with a higher content of large LDL in girls (17.8% vs. 13.5%; p < 0.001) and a significant, lower content of small LDL (1.7% vs. 3.4%; p = 0.002). When obesity was considered, we observed that regardless of being overweight, girls had higher large and small LDL than boys. However, when a large to small LDL ratio was calculated, girls with no excess weight had higher values than boys with no excess weight (10.6 vs. 9.3; p = 0.038), and these had lower LDL ratio than overweight boys (9.3 vs. 13.5; p = 0.016). On the other hand, boys had higher HDL 2 content than girls (8.9% vs. 8.0%; p = 0.017), which was associated with increased large HDL values in boys (1.9% vs. 1.7%; p = 0.047). Regression analysis was performed according to gender, the sum of very low-density lipoprotein (VLDL) + intermediate density lipoprotein (IDL) C + IDL B subfractions was adjusted for age and body mass index (BMI), showing that girls had lower atherogenic lipid profile (β = 0.987; CI = 0.977-0.998; p = 0.017) than boys. When the regression analysis was performed according to BMI, large LDL in adolescents with no excess weight presented a lower atherogenic lipid profile (β = 1.040; CI = 1.000-1.082; p = 0.049), adjusted for age and sex, than overweight adolescents.
Conclusion: Regardless of excess weight, girls showed a cardioprotective profile more associated with a favorable distribution of LDL subfractions than boys, reinforcing the relevance of evaluating qualitative aspects of lipoproteins in addition to the traditional cholesterol content.
The increasing trends of obesity in adolescents remain a serious epidemiological concern worldwide [1], especially considering that overweight and obesity are associated with multiple adverse health outcomes throughout the life course. Obesity is linked to an increased risk of hypertension and other non-communicable diseases like Diabetes Mellitus (DM) and coronary heart disease [2], resulting in the risk of premature death [3]. This scenario is usually accompanied by a low-grade inflammatory and oxidative process that favors the development of insulin resistance and promotes significant changes in lipid and glucose metabolism [4].
These events contribute to the onset of Dyslipidemia (DLP) and early atherosclerotic lesions [5]. In addition to DLP, the mechanism linking obesity with atherosclerosis process includes insulin resistance [Homeostasis Assessment Model- Insulin Resistance (HOMA-IR), Quantitative Insulin sensitivity Check Index (QUICKI score)], increased visceral and ectopic fat deposition (visfatin), unbalancing in adipokynes (leptin, adiponectin, resistin), positive stimulus to inflammation as C-reactive protein (PCR), factor nuclear kappa B (NF-kB), Tumor necrosis factor-alpha (TNF-alpha), monocyte chemotactic protein-1 (MCP-1), procoagulation and hypofibrinolysis factors as fibrinogen, Willebrand factor, plasminogen activator inhibitor-1 (PAI-1) and oxidative stress (oxidized low-density lipoprotein, malondialdeyde) [5]. Although classical lipids and markers described in the above process are very well described in atherosclerosis, studies monitoring lipoprotein subfractions have expanded the knowledge on the role of lipoproteins on atherosclerosis in the last decade [6,7]. Although obesity in adolescence can induce DLP, and possibly early fat deposition in arteries, monitoring an unbalanced lipid profile is not routine in clinical care.
Although high-density lipoprotein cholesterol (HDL-c) is considered an anti-atherogenic marker, the role of HDL subfractions remains a controversial issue. Pirillo, et al. (2013) [8] described that large HDL is associated with a more effective cholesterol reverse transport (CRT) than small HDL, while the last one was associated with improvement in free radical quenching [9] and uptake of cholesterol by adenosine triphosphate-binding cassette transporter A1 (ABCA-1) in macrophafes [10].
Small LDL and small HDL are lipoprotein subfractions with a lower cholesterol content and are therefore smaller and denser. For this reason, small LDL infiltrates more into the intima tissue, is increased in the tissue, and increases inflammatory response, thus being more atherogenic than large LDL. Small HDL also has worse CRT and antioxidant action, being less cardioprotective when compared to large HDL. On the other hand, large LDL and large HDL are lipoprotein subfractions with a higher cholesterol content and consequently are larger and less dense particles. For this reason, large LDL infiltrates less into the intima tissue, is decreased in the tissue, and decreased inflammatory response, being less atherogenic than small LDL. Likewise, large HDL has better CRT, functionality, and better antioxidant and anti-inflammatory action, being more cardioprotective than small HDL.
Obesity is the major risk factor associated with altered HDL subfraction profile in adolescents with type 2 diabetes mellitus. However, there is evidence that lean adolescents without insulin resistance tend to present higher levels of large HDL than obese subjects with insulin resistance in certain populations [11]. Yet, lack of studies describing the distribution of HDL subfractions of healthy adolescents in populational studies. Obese adolescents are usually candidates for increased risk of cardiovascular disease (CVD) in adulthood. Some studies have shown significantly higher large HDL subfractions in the obese population [12]. According to this study, low levels of large HDL subfraction can be considered a potential predictor for developing CVD in the future [12,13]. A significant improvement in LDL size and large HDL subfraction after 12 weeks of follow-up was observed in a randomized controlled clinical trial based on lifestyle intervention including obese Latino adolescents. Additionally, the LDL phenotype changed for a less atherogenic profile [14].
Similarly, to mechanisms observed in adults, there are sex-dependent differences in lipid metabolism that may explain, at least in part, the differences in the incidence and prevalence of CVD, and its morbidities, between boys and girls. Although women have nearly one decade of protection from first myocardial infarction compared to men due to the hormonal effect of estrogen on cholesterol metabolism [15] the traditional lipid profile does not consider sex differences for cardiovascular risk based on lipid profile in adolescents.
The pioneer Bogalusa study confirmed that the atherosclerotic process begins in childhood and adolescence [16], and more recently, that the quantification of LDL and HDL lipoprotein particle size has been proposed as a predictor of CVD incidence, independently of cholesterol content in LDL and HDL [17,18]. Therefore, it is plausible that the analysis of lipoprotein subfractions can identify adolescents vulnerable to higher cardiovascular risk.
Thus, we investigate the potential differences between traditional lipid markers and LDL and HDL subfractions in a sample of Brazilian adolescents, which is representative at the population level. In addition, we evaluated the impact of obesity and sex on the distribution of lipoprotein subfractions, aiming to identify subgroups of adolescents in the population with potential increased cardiovascular risk.
Subjects
The individuals included in the study comprise a subsample of the 2015 Health Survey of São Paulo (ISA-Capital 2015) [19]. The study was approved by the Ethics Committee, and all participants and their parents were fully informed and signed a written consent form before participation in the survey.
The population of reference for ISA-Capital 2015 refers to individuals over 12 years of age living in permanent private households in the urban area of the city of São Paulo, Brazil, the largest municipality in the country. The definition of the population was based on census sectors obtained in the 2010 Brazilian Census. Stratified sampling was used by drawing clusters in two stages: census tracts and households.
Three study domains were defined in sample planning: geographical area, age, and sex groups. Sampling strata were based on catchment areas of the five health coordination regions in the city of São Paulo: North, Midwest, Southeast, South, and East. Age and sex groups were defined through the distribution of the population into six clusters: male and female adolescents (12 to 19 years old), adults (20 to 59 years old), and elderly individuals (over 60 years old). This study included only adolescents in the sample. Details of the sample were previously described [19].
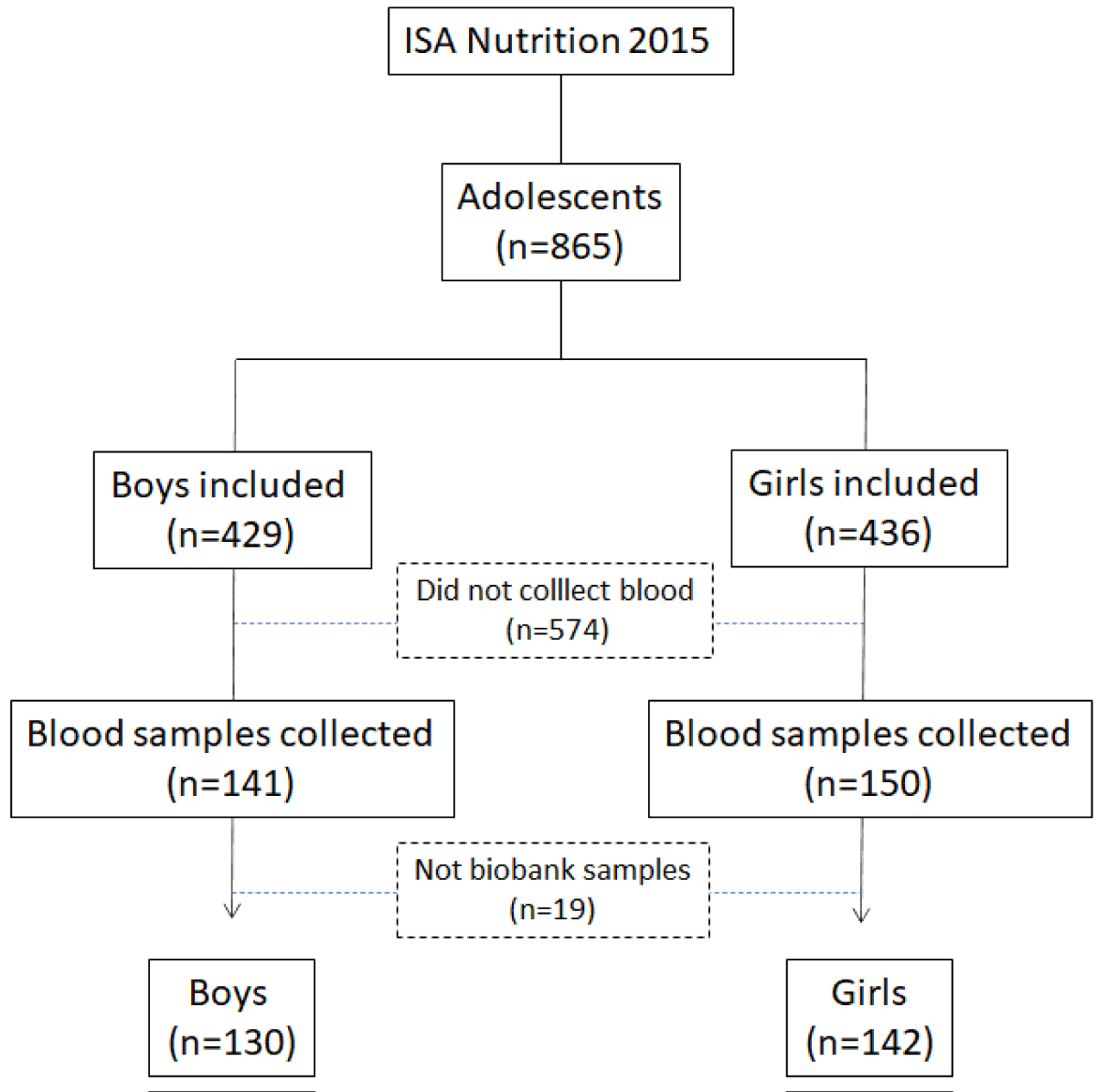
The 2015 Health Survey of São Paulo focusing on Nutrition (ISA-Nutrition 2015) was based on a subsample of the ISA-Capital 2015, selected for application of 24-hour recall (24HR) and additional health assessments, including 300 adolescents. The 300 participants per domain were planned to allow the estimation of proportions of changes/differences of 0.50, with a sampling error of seven percentage points, considering a 95% confidence level of the population value being within the lower and upper limits (Figure 1).
Figure 1: Flowchart of selection of adolescent subjects participating in ISA Nutrition 2015.
Anthropometric measurements
The anthropometric data collection methodology was previously published [19]. Participants’ weight and height were measured in triplicate, with participants barefoot and wearing light clothing. Weight was measured in kilograms using a calibrated digital platform scale (Tanita®, model HD-313, Arlington Heights, IL, USA, maximum capacity of 150 kg, and accuracy of 100 g). A portable stadiometer (Seca®, model 208, Cotia, SP, Brazil, maximum measurement of 200 cm, and precision of 0.1 cm) fixed on a smooth wall, without a plinth, was used to measure height in centimeters.
Weight, height, and waist circumference were collected according to WHO guidelines (2000) [20]. For weight measurement, the subjects were asked to stand in the center of the scale platform, in a vertical position with the feet parallel and together, and with the arms placed along the body. For height measurement, subjects were positioned in the Frankfurt plane, with their heels, calves, buttocks, and shoulders, and the back of their heads touching the vertical surface of the stadiometer. Participants were asked to remove any adornments that might interfere with the measurement.
The waist circumference was measured with an inextensible measuring tape, positioned above the midpoint, between the last costal arch and the iliac crest of the standing participants, during expiration. The average values of weight and height of each participant were used to calculate BMI and were calculated according to weight/height(2). BMI values were used to classify nutritional status, according to the WHO criteria [21] for adolescents through the growth curves (BMI values
The weight, height, and waist circumference of adolescents were measured and the values of BMI and BMI z-score were calculated. After obtaining the BMI value, the classification was made according to the BMI-for-age growth curves for adolescents (CDC, 2007). The BMI results were presented in this study according to the non-overweight group (underweight and normal weight) and the overweight group (overweight and obesity).
Assessment of physical activity practice
Physical activity was assessed using the International Physical Activity Questionnaire, validated for the Brazilian population [22,23]. The criteria for classifying physical activity were: 1) physical activity at work, 2) walking as a means of transportation, 3) household chores, 4) recreation, and 5) time spent sitting. After completing the questionnaire, a score was calculated and the subjects were classified as “meeting recommendation” or “not meeting recommendation”, according to the most recent proposal for the assessment of physical activity [24].
Assessment of alcohol consumption
Alcohol consumption was measured using the Alcohol Use Disorders Identification Test [25]. This instrument addresses alcohol dependence, harmful consumption, and hazardous alcohol consumption, being validated for the Brazilian population [26]. The AUDIT was developed by WHO as a screening instrument for alcohol-related disorders in general. Its 10 questions refer to the previous 12 months and address alcohol dependence (questions 4–6), harmful consumption (questions 7 – 10), both as defined by ICD-10, and hazardous alcohol consumption, considered as a threshold of consumption which predicts future harm (questions 1 – 3). Questions about the frequency of drinking, quantity in a typical day, binge drinking, unable to stop, failing to do expected things, feeling guilty, blackouts, and injuring others [25].
Blood pressure measurement
Blood pressure was measured by trained nurses using an automatic device (Omron, model HEM-712C, Omron Healthcare, Inc., Kyoto, Japan). Two blood pressure measurements were taken using an appropriate cuff for the participants’ arm circumference. Measurements were taken after the participants rested for 5 minutes in a sitting position, with the arm supported at heart level. Blood pressure was initially measured on the right arm and the second measurement was performed in the left arm 1 minute after the first measurement. An additional measurement was taken on the arm with the highest value. If there was a difference greater than 10% between the two measurements of the same arm, a third measurement was performed. The results of systolic and diastolic pressures are expressed as averages, in mmHg.
Laboratory analyses
Blood samples were collected during the second visit, after 12-hour fasting, 72 hours without consumption of alcoholic beverages, and no intense physical activity before and on the day of blood collection. The blood collection visit took place approximately 48 days after the first visit and was conducted by trained nurses, who performed venous blood sampling (~30 mL) using Vacutainer tubes. At this stage, another informed consent was signed by participants, and the use of medications and/or supplements was recorded. Blood samples were processed at the Laboratory of Nutritional Genomics and Inflammation of the School of Public Health at the University of São Paulo, Brazil. Nine aliquots per participant were immediately sent to the laboratory for analysis, including total cholesterol (TC), LDL-c, HDL-c, and triglycerides (TG) performed in an automated system (Cobas; Roche Diagnostics GmbH, Mannheim, BW, Germany). Samples for analysis of lipoprotein subfractions were stored at -70 oC. Serum VLDL values were calculated by dividing triglyceride values by five. The prevalence of DLP in adolescents was based on recommendations of the Brazilian Society of Cardiology [27].
Quantification of the LDL and HDL Lipoproteins Subfractions
LDL and HDL particle sizes were determined by the Lipoprint® System (Quantimetrix Corporation). First, 25 µL of plasma and 200 µL for LDL analysis or 300 µL for HDL analysis of a gel containing lipophilic dye were pipetted. After homogenization (7x), the sample applied to the polyacrylamide gel went through the photopolymerization process (30 min), followed by running in an electrophoresis buffer. The bands showed the relative amount of lipoprotein particles per sample, in decreasing order of particle size. From the LDL subfractions kit were analyzed 1 VLDL band, IDL A, B, and C, and 7 LDL subfractions. LDL 1 and 2 were classified into larger and less dense particles. From the sum of LDL, 3 to 7 subfractions were identified as the smaller and denser particles. After the application of the cut-off point based on total LDL size, it was identified that phenotype A ≥ 26.51 nm - less atherogenic), and phenotype B (< 26.5 nm - atherogenic). For HDL subfractions, the kit allows to identify 10 HDL subfractions in which HDL 1 to 3 were classified as large HDL, HDL 4 to 7 as intermediate, and HDL 8 to 10 as small. Based on large and small subfractions, were calculated ratios: large LDL/small LDL and large HDL/ small HDL. All results were expressed in percentage of area under the curve.
Statistical analyses
The distributions of variables were assessed using the Kolmogorov-Smirnov test (p > 0.05). Qualitative variables were presented in absolute value (n) followed by their respective percentage (%), and tested using the chi-square test (x2); whilst quantitative variables were presented in mean and standard deviation. Variables without normal distribution were analyzed using the Mann-Whitney test. Univariate and multivariate linear regression models were tested, including adjustments for age, sex, IMC, and physical activity. The analyses were performed in the SPSS 16.0 version, adopting a significance level of p < 0.05.
Table 1 describes the demographic and biochemistry profile of 272 Brazilian adolescents included in the study. The mean age was 15.2 ± 2.2 years, and 47.8% (n = 130) were boys. We observed that more boys than girls declared smoking (p = 0.068). Both groups showed a high prevalence of DLP (> 54% in the total sample), but boys presented higher inadequate values of blood pressure than girls (25% vs. 14.4%; p = 0.029), confirmed by elevated values of systolic blood pressure (SBP) (p < 0.001). On the other hand, girls had higher central adiposity levels, estimated by waist circumference, than boys (11.5% vs. 15.3%; p = 0.041). Although girls had lower levels of glucose, TG levels were higher than boys (89.1 mg/dL vs. 83.4 mg/dL; p < 0.044). Alcohol consumption was assessed through a questionnaire that addresses not only daily consumption but also dependence on alcohol use. Our results showed that 91.9% of adolescents never consumed alcohol, which was expected due to their age group.
| Variables | Total Adolescents | Boys | Girls | p |
| n = 272 | n = 130 | n = 142 | ||
| Age, years (m/SD) | 15.2 (2.2) | 15.3 (2.2) | 15.1 (2.2) | 0.454 |
| Race/skin color (n, %) | 113 (44.5) | 55 (45.1) | 58 (43.9) | 0.855 |
| White | ||||
| Brown + Black | 141 (55.5) | 67 (54.9) | 74 (56.1) | |
| Smoking (n, %) | 258 (95.2) | 120 (92.3) | 138 (97.9) | 0.063 |
| Never | ||||
| Yes | 8 (3.0) | 7 (5.4) | 1 (0.7) | |
| Alcohol intake (n, %) | 248 (91.9) | 116 (89.2) | 132 (94.3) | 0.129 |
| Never | ||||
| Yes | 22 (8.1) | 14 (10.8) | 8 (5.7) | |
| Physical activity (n, %) | 133 (48.9) | 0.635 | ||
| Yes | 63 (48.5) | 70 (49.3) | ||
| No | 135 (49.6) | 66 (50.8) | 69 (48.6) | |
| Current diseases (n, %) | ||||
| Hypertension | 52 (19.5) | 32 (25) | 20 (14.4) | 0.029 |
| Dyslipidemia | 146 (54.1) | 70 (54.3) | 76 (53.9) | 0.952 |
| Anthropometric measurements (m/SD) | ||||
| Weight (kg) | 59.2 (15.8) | 58.9 (15.5) | 59.45 (16.1) | 0.904 |
| Waist circumference (cm) | 77.2 (13.7) | 75.23 (11.5) | 79.10 (15.3) | 0.041 |
| BMI (kg/m2) (n/%) | 0.027 | |||
| Non-overweight | 186 (68.4) | 96 (73.4) | 90 (63.4) | 0.784 |
| Overweight | 80 (29.4) | 30 (23.1) | 50 (35.2) | 0.549 |
| Biomarkers (m/SD) | ||||
| Glucose (mg/dL) | 89.9 (7.3) | 91.6 (7.2) | 88.27 (7.1) | <0.001 |
| SBP (mm/Hg) | 114.5 (11.3) | 117.15 (11.6) | 112.06 (10.5) | <0.001 |
| DBP (mm/Hg) | 68.9 (8.9) | 68.5 (9.7) | 69.24 (8.1) | 0.478 |
| TG (mg/dL) | 86.4 (42.4) | 83.40 (45.6) | 89.12 (39.1) | 0.044 |
| TC (mg/dL) | 143.9 (29.8) | 140.74 (27.8) | 146.73 (31.3) | 0.257 |
| LDL-c (mg/dL) | 81.9 (25.3) | 79.90 (22.5) | 83.71 (27.6) | 0.501 |
| HDL-c (mg/dL) | 44.5 (11.4) | 44.13 (10.4) | 44.81 (12.3) | 0.632 |
| Results are presented in absolute values (n) and percentage (%) or mean (standard deviation). BMI: Body Mass Index; WC: Waist Circumference; TC: Total Cholesterol; LDL-c: Low-density Lipoprotein Cholesterol; HDL-c: High-density Lipoprotein Cholesterol; TG: Triglycerides; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure. Differences between sexes were tested by Qui-square test or t - student test. The significance level was p < 0.05. | ||||
There were no statistically significant differences in the prevalence of hypercholesterolemia (16.5%), hypertriglyceridemia (37.0%), and low HDL (55.5%) between sexes (p > 0.05). None of the adolescents self-reported having DM, which was confirmed by adequate glucose levels and non-regular use of hypoglycaemic medication. The analysis of LDL subfractions showed increased content of LDL 2 in girls (5.7% vs. 5.1%; p = 0.047). This result was associated with higher content of large LDL in girls (17.8% vs. 13.5%; p < 0.001), and consequently lower level of small LDL (1.7% vs. 3.4%; p = 0.002) (Table 2).
| Table 2: LDL subfractions and size according to sex among adolescents participating in ISA-Nutrition 2015. | |||
| Variables (%) | Boys (n = 130) | Girls (n = 142) | p |
| VLDL | 25.2 (8.7) | 25.8 (9.9) | 0.340 |
| IDL-C | 8.8 (5.9) | 9.5 (5.4) | 0.510 |
| IDL-B | 11.9 (5.8) | 14.4 (6.5) | 0.086 |
| IDL-A | 9.7 (4.1) | 10.1 (4.5) | 0.370 |
| LDL 1 | 10.7 (4.2) | 10.0 (4.7) | 0.122 |
| LDL 2 | 5.1 (3.6) | 5.7 (3.9) | 0.047 |
| LDL 3 | 1.6 (1.7) | 1.8 (2.5) | 0.155 |
| LDL 4 | 0.6 (1.2) | 0.5 (1.3) | 0.374 |
| LDL 5 | 0.2 (0.9) | 0.2 (0.7) | 0.558 |
| LDL 6 | 0.1 (0.6) | 0.1 (0.3) | 0.248 |
| LDL 7 | 0.1 (1.1) | 0.0 (0.3) | 0.911 |
| Large LDL | 13.5 (6.9) | 17.8 (5.8) | <0.001 |
| Small LDL | 3.4 (5.7) | 1.7 (2.5) | 0.002 |
| LDL ratio | 4.6 (3.9) | 10.5 (6.2) | 0.524 |
| LDL size (nm) | 267.8 (6.9) | 268.7 (5.9) | 0.084 |
| Phenotype A (n, %) | 86.0 (66.2) | 107.0 (75.4) | 0.097 |
| LDL-c: Low-density Lipoprotein Cholesterol; LDL ratio: Low-Density Lipoprotein Large/Low-Density Lipoprotein Small. Results are presented in mean (standard deviation) and analyzed by the Mann-Whitney U test. Categorical variables are presented in absolute values (n) and frequency (%), and analyzed by Chi-square test. The significance level was p < 0.05. | |||
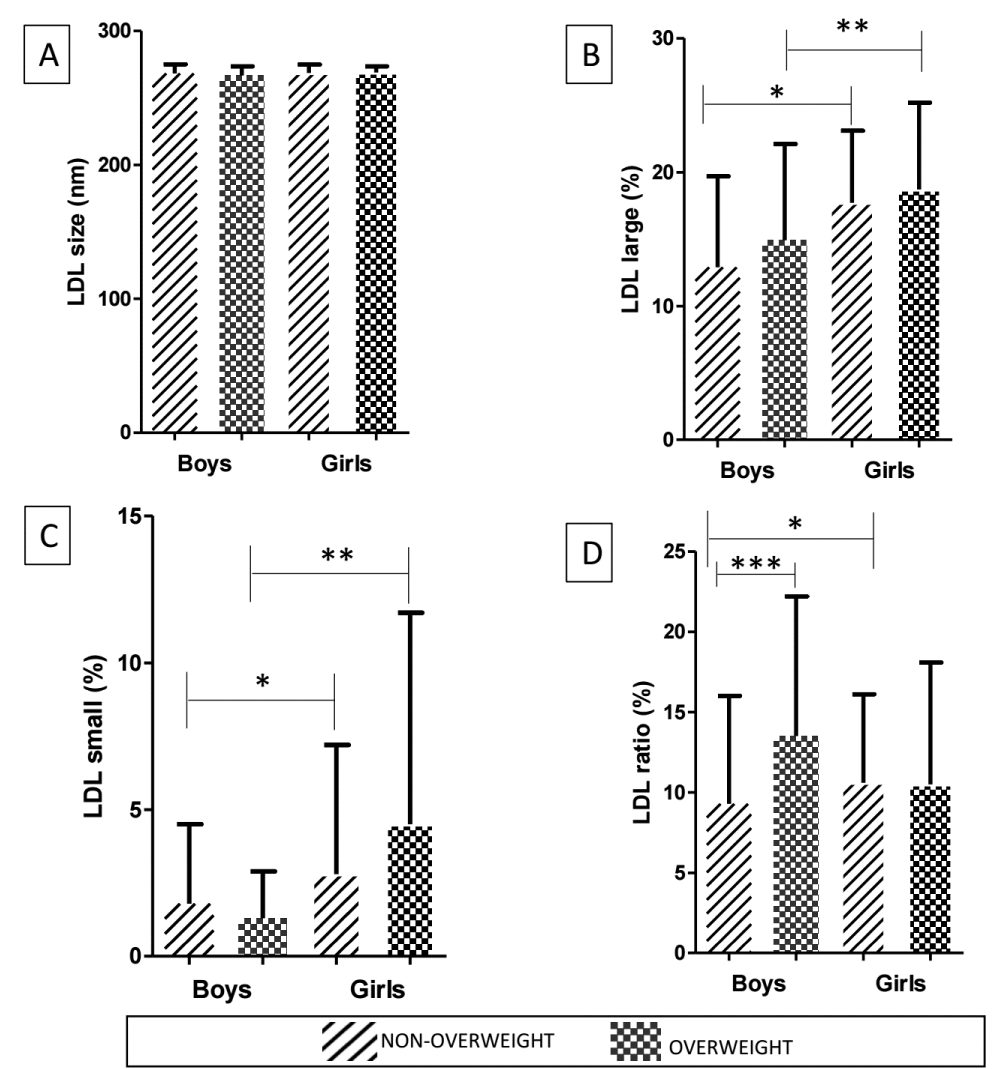
In addition, our study evaluated IDL-C (%) (boys 8.8 vs. girls 9.5; p = 0.510), IDL-B (%) (boys 11.9 vs. girls 14.4; p = 0.086), and IDL-A (%) (boys 9.7 vs. girls 10.1; p = 0.370). When obesity was considered, we observed that regardless of being overweight, girls had higher large and small LDL than boys. However, when LDL ratio was calculated, non-overweight girls presented higher values than non-overweight boys (10.6 vs. 9.3; p = 0.038), and these had lower content of LDL ratio than overweight boys (9.3vs. 13.5; p = 0.016) (Figure 2).
Figure 2: Comparison of LDL characteristics according to sex and nutritional status of adolescents. LDL size (%) [A], large LDL (%) [B], small LDL (%) [C], LDL ratio (%) [D]. Data are presented as mean (standard deviation) and analyzed by the Mann-Whitney U test. LDL, Low-density lipoprotein; LDL ratio, Large LDL/small LDL. (*) = p < 0.05 comparison between non-overweight boys and non-overweight girls. (**) = p < 0.05 comparison between overweight boys and overweight girls. (***) = p < 0.05 comparison between non-overweight boys and overweight boys.
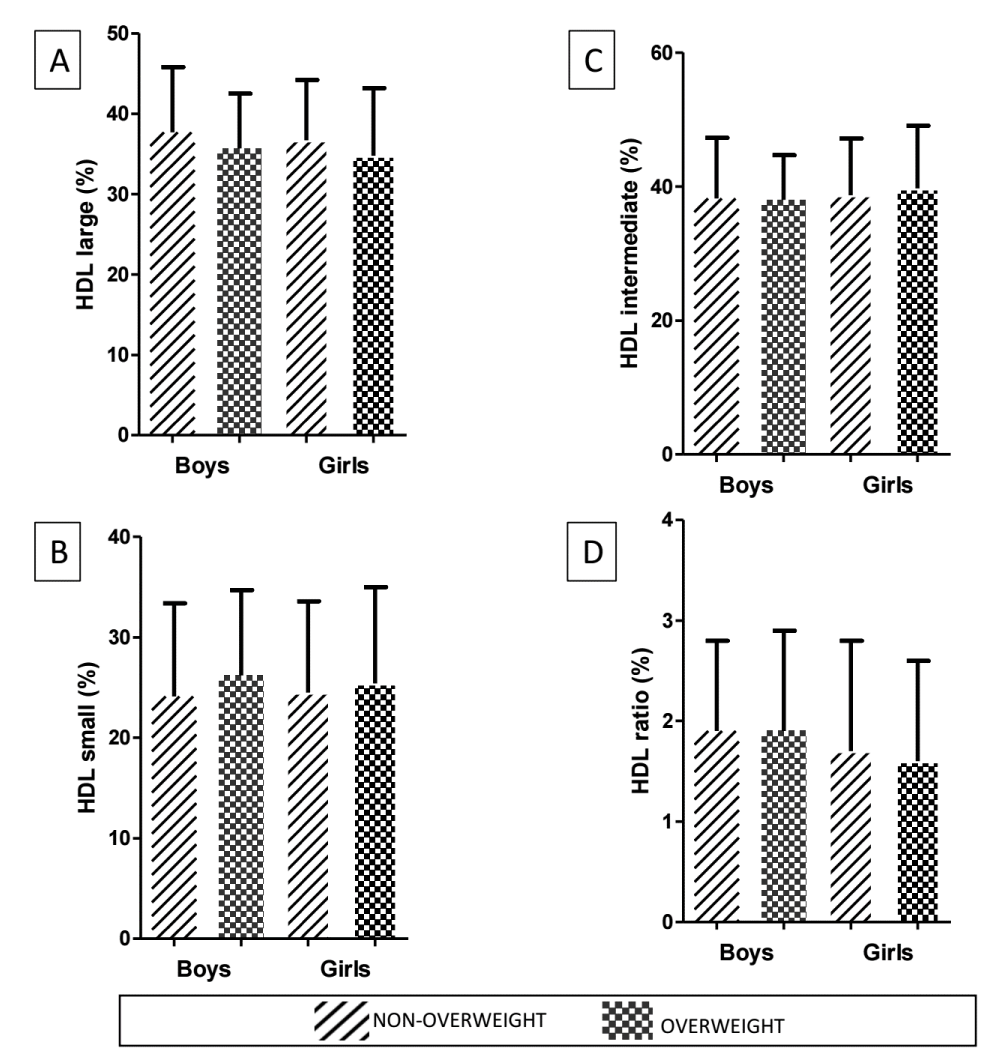
Contrarily, boys had a higher content of HDL 2 than girls (8.9% vs. 8.0%; p = 0.017), which was associated with increased HDL ratio values in boys (1.9% vs. 1.7%; p = 0.047) (Table 3). There were no statistically significant differences in HDL ratio according to sex and nutritional status (Figure 3).
| Table 3: HDL subfractions according to sex among adolescents participating in ISA-Nutrition 2015. | |||
| Variables (%) | Boys (n = 130) | Girls (n = 142) | p |
| HDL1 | 21.5 (6.1) | 21.6 (6.1) | 0.890 |
| HDL2 | 8.9 (3.3) | 8.0 (2.2) | 0.017 |
| HDL3 | 6.7 (2.5) | 6.5 (2.3) | 0.364 |
| HDL4 | 8.9 (3.7) | 9.0 (3.5) | 0.321 |
| HDL5 | 8.4 (3.4) | 8.6 (3.2) | 0.522 |
| HDL6 | 14.5 (5.8) | 14.8 (5.7) | 0.712 |
| HDL7 | 6.3 (3.5) | 6.6 (3.5) | 0.340 |
| HDL8 | 7.0 (2.7) | 7.4 (3.1) | 0.510 |
| HDL9 | 5.8 (2.8) | 6.6 (3.2) | 0.086 |
| HDL10 | 11.9 (8.9) | 11.0 (9.6) | 0.418 |
| Large HDL | 37.1 (7.7) | 36.1 (7.7) | 0.370 |
| Intermediate HDL | 38.1 (8.5) | 39.0 (8.6) | 0.392 |
| Small HDL | 24.7 (9.1) | 24.9 (9.1) | 0.945 |
| HDL ratio | 1.9 (0.9) | 1.7 (1.1) | 0.047 |
| HDL-c: High-Density Lipoprotein Cholesterol; HDL ratio, large HDL/small HDL. Results are presented in mean (standard deviation), and analyzed by Mann-Whitney U test. The significance level was p < 0.05. | |||
Figure 3: Comparison of HDL characteristics according to sex and nutritional status of adolescents. Large HDL (%) [A], intermediate HDL (%) [B], small HDL (%) [C], HDL ratio (%) [D]. Data are presented as mean (standard deviation) and analyzed by the Mann-Whitney U test, and the significant level adopted was set at p < 0.05. HDL: High-Density Lipoprotein; HDL ratio, Large HDL / small HDL High-Density Lipoprotein large / High-Density Lipoprotein small. There were no statistically significant differences between groups according to sex and nutritional status.
The regression analysis was performed according to sex, the sum of VLDL + IDL C + IDL B subfractions adjusted by age and IMC, showing that girls had less atherogenic lipid profile (β = 0.987; IC = 0.977 - 0.998; p = 0.017) than boys. When regression analysis was performed according to BMI, the LDL large in non-overweight adolescents showed less atherogenic lipid profile (β = 1.040; IC = 1.000-1.082; p = 0.049), adjusted by age and sex, than overweight adolescents.
The traditional lipid profile and lipoprotein subfractions can reflect many genetic and environmental determinants and their relationships. In the present study, we investigated the impact of sex and nutritional status on lipid metabolism. Our results showed that girls, regardless of increased visceral adiposity and high TG levels, had better LDL and HDL subfraction profiles than boys.
Previously, our group evaluated the lipid profiles of adolescents with different nutritional status. Obesity was associated with increased content of TC and LDL-c [28]. Physical activity was assessed through a questionnaire covering physical activity at work, walking as a means of transportation, household chores, recreation, and time spent sitting. Our results showed that 48.9% were “meeting recommendation” and 49.6% were “not meeting recommendation”. Considering teenagers walking to school and other places, we found that 68.4% were non-overweight. Regarding LDL subfractions, after performing multiple linear regression, the results (not presented in tables) showed that the female gender was positively associated with large LDL (β = 0.118), after adjustments for BMI and physical activity. This model was able to explain 1.2% of the large LDL variability (R2 = 0.012).
More recently, the prevalence of DLP in Brazilian adolescents was evaluated in 77,833 subjects. Similarly, to findings in our study, hypertriglyceridemia was diagnosed in 15.7% of adolescents, followed by hypercholesterolemia (26.8%), and low HDL-c (40.8%) [29]. Additionally, other studies conducted in Brazil identified a high prevalence of dyslipidemia among adolescents [30]. In the present study, we observed simultaneously increased visceral adiposity and hypertriglyceridemia in girls than in boys. High-fat deposition in visceral adiposity promotes positive stimulus to inflammatory response and oxidative stress, both essential events to the atherosclerotic process [31]. Although atherosclerosis and its clinical outcomes tend to occur later in the life course, there is a consensus that it develops since early childhood and adolescence. Yet, a major part of the evidence on DLP in adolescents is based on the analysis of data from adults [32]. Therefore, it is important to identify the sensitivity of lipid markers and their ability to predict cardiovascular risk in adolescents.
In addition to sex-dependent hypertriglyceridemia, our results describe qualitative aspects of LDL and HDL subfractions, showing a cardioprotective profile among girls, characterized by increased levels of large LDL and large to small LDL ratio. Numerous studies provide evidence that small dense LDL particles have the greatest atherogenic potential due to easier migration into arterial intima, longer retention in sub endothelium through the link to proteoglycans, and higher oxidative susceptibility [33]. During adolescence, both, girls and boys, experience significant changes in sex hormones-dependent lipid metabolism [34]. Considering that 100% of girls in our study are in the pubertal phase, the sexual hormones can, at least in part, explain the more cardioprotective profile observed in this group. The estrogen promotes positive stimulus to Apolipoprotein (Apo) AI synthesis and LDL receptor expression [34]. Furthermore, recently, Choi, et al. (2022) verified that girls presented higher LDL size than boys (27.1 ± 0.7 vs. 26.6 ± 0.8, p = 0.0012) [35]. Despite that, the size of this lipoprotein was not related to estrogen, but a negative association was observed between free androgen index and LDL size in boys (r = -0.273, p = 0.026) [35]. Other studies have identified the relationship between small LDL particles and CVD risk [36]. However, a systematic review based on 24 studies concluded that there is a lack of robust evidence to confirm whether LDL subfractions add benefits to traditional lipid profile [37]. Nevertheless, it is important to interpret this conclusion with caution, since most studies were conducted with adults and obtained results using different methods, like magnetic resonance spectroscopy (MNR) [38], ion mobility [39], and combined density gradient ultracentrifugation and nondenaturing gradient gel electrophoresis (GGE) [40], which present only moderate concordance levels [41].
Similarly, the relationship between LDL subfractions and CVD incidence was investigated in 27,673 women from the Women’s Health Study over 11 years of follow-up, showing a significant association between total LDL and small LDL (p < 0.001) [42]. Our results expand the traditional information provided by lipid profile, allowing us to identify additional qualitative details on LDL subfractions, and highlighting the occurrence of less atherogenic risk in girls, regardless of waist circumference and hypertriglyceridemia. Furthermore, we found higher values of LDL ratio (large LDL/small LDL) in non-overweight girls in comparison to boys.
Conversely to the cardioprotective profile of LDL subfractions in favor of the girls, boys presented a high HDL ratio (large HDL/small HDL), directly linked to increased values of HDL2. It is well known that high HDL-c levels are associated with decreased cardiovascular risk; however, in the last years, some parameters of HDL functionality (anti-platelets, anti-inflammatory, antioxidant, and microRNA transport) emerged, expanding the cardioprotective role of HDL [43]. Despite that, reverse cholesterol transport (RCT) remains the most studied antiatherogenic mechanism attributable to HDL. During RCT, HDL particles uptake free cholesterol from the peripheral tissues, such as endothelial and adiposity cells, and transfer it to the liver, stimulating the excretion of cholesterol by bile acids [44].
In 2017, Christensen, et al. described a comprehensive lipid profile of children with and without familial hypercholesterolemia (FH). Although FH children present increased atherogenic lipoproteins, the unfavorable profile of HDL subfractions indicated impaired RCT [45]. Previous studies based on the size exclusion chromatography technique isolated 15 lipoprotein subfractions by size. The authors found significantly lower phospholipid content in large HDL in obese adolescents [46]. The reduction in these species was associated with high vascular stiffness in boys [47].
In our study, we did not analyze the composition of lipids according to HDL subfractions, but we cannot exclude the possibility of a worse lipid profile due to large HDL. Boys, in comparison to girls, presented higher self-reported hypertension, confirmed by higher systolic blood pressure and glucose levels. These characteristics combined can modify the functionality of HDL, regardless of its subfractions [48]. Although results from global studies found that dyslipidemia occurs in more than 70% of children and adolescents [49], highlighting the urgent need to improve the early diagnosis and management of dyslipidemia in these subjects, analysis of lipoprotein subfractions remains limited to a small number of studies. We also investigated the role of overweight on HDL subfractions, and interestingly, there were no statistically significant differences. Similarly, HDL subfractions were analyzed in hypercholesterolemic and healthy children and adolescents. Although sex-dependent differences were not observed, hypercholesterolemia was associated with a high content of large HDL [50].
This study contributes to the evidence on associations between sex, nutritional status, and subfractions of HDL and LDL in Brazilian adolescents. Although results from global studies found that DLP occurs in more than 70% of children and adolescents [51], highlighting the urgent need to improve the early diagnosis and management of DLP in early life stages, the analysis of lipoprotein subfractions remains limited to a small number of studies. We investigated the role of sex and overweight on lipid and lipoprotein subfractions, but isolated and combined effects of genetic, environmental, and epigenetic factors may act on lipid metabolism.
The complex relationship between sex, overweight, and lipid metabolism includes additional mechanisms modulated by insulin such as accumulation of visceral adiposity and hypertriglyceridemia and blood pressure control. Although glucose metabolism does not indicate abnormal values (cutoff point 99.0 mg/dL), the high waist circumference and triglyceride levels observed in girls suggest the presence of insulin resistance. We analyzed insulin or some insulin resistance index, and we identified a hypertriglyceridemia girls group (10.6%) (cutoff point ≤ 130.0 mg/dL) [52], and 21.8% of girls (12 – 15 years old) and 13.4% of girls (16 years - 19 years old) have waist circumference with values suggesting increased cardiovascular risk. Therefore, monitoring LDL and HDL subfractions can indicate early alterations in lipid metabolism in adolescents, particularly in overweight boys.
Finally, our study presents some limitations. First, glucose levels in boys were higher than in girls, but we cannot confirm insulin resistance due to the absence of insulin measurements. The negative impact of insulin resistance on lipid metabolism is very well described in the literature [53] but its role on lipoprotein subfractions remains an issue to be explored. Second, obesity induces impaired glucose and lipid response as demonstrated previously, but adolescents were grouped according to excess weight and obesity. Although the inclusion of overweight adolescents may potentially reduce the differences between groups, we decided to explore the associations with unhealthy weight to identify early cardiovascular risks among adolescents. Third, the overweight prevalence in girls was 67% higher than in boys. Yet, it is important to highlight the populational-based study design of our study, which represents a strength of our results in the association of lipoprotein subfractions with sex and nutritional status among adolescents. Another point, our results showed that 91.9% of adolescents never consumed alcohol. Since alcohol consumption was assessed through a questionnaire that addresses dependence on alcohol use (not occasional consumption), perhaps the number was too high.
In conclusion, our results support that girls, regardless of overweight, presented a more cardioprotective profile linked to a favorable distribution of LDL subfractions than boys in Sao Paulo City, Brazil. Thus, further studies investigating adolescents in other populations should be performed, including analyses that include individuals with distinct clinical conditions.
The authors would like to thank Rosana A. M. S. Freitas for the technical assistance in the analysis of subfractions lipoproteins, and Maria C. P. de Freitas for statistical support. M.C.P. contributed to the statistical analysis.
Authors’ contributions
M.N.A. contributed to performing statistical analyses and writing, and N.R.T.D. contributed to the study design, critical review, and writing. R.M.F., M.M.R., and F.M.S. contributed to the critical review and writing.
Ethical approval and participation consent
No 5.059.428/2020
Data availability: The data can be made available from authors upon request.
Funding information
Grants received from the State of São Paulo Research Foundation FAPESP (No 2017/05125-7), and Higher Education Personnel Improvement Coordination (CAPES) (No 88887.487140/2020-00).
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Aug 30;384(9945):766-81. doi: 10.1016/S0140-6736(14)60460-8. Epub 2014 May 29. Erratum in: Lancet. 2014 Aug 30;384(9945):746. PMID: 24880830; PMCID: PMC4624264.
- Abdalmaleki E, Abdi Z, Isfahani SR, Safarpoor S, Haghdoost B, Sazgarnejad S, Ahmadnezhad E. Global school-based student health survey: country profiles and survey results in the eastern Mediterranean region countries. BMC Public Health. 2022 Jan 19;22(1):130. doi: 10.1186/s12889-022-12502-8. PMID: 35045855; PMCID: PMC8767753.
- World Health Organization (WHO). Impacting in premature death in adult life; World Health Organization: Geneva, Switzerland, 2016.
- Libby P, Hansson GK. From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week. J Am Coll Cardiol. 2019 Sep 24;74(12):1594-1607. doi: 10.1016/j.jacc.2019.07.061. PMID: 31537270; PMCID: PMC6910128.
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006 Dec 14;444(7121):875-80. doi: 10.1038/nature05487. PMID: 17167476.
- Lee S, Gungor N, Bacha F, Arslanian S. Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care. 2007 Aug;30(8):2091-7. doi: 10.2337/dc07-0203. Epub 2007 May 2. Erratum in: Diabetes Care. 2007 Oct;30(10):2763. PMID: 17475936.
- Pérez-Méndez O, Torres-Tamayo M, Posadas-Romero C, Vidaure Garcés V, Carreón-Torres E, Mendoza-Pérez E, Medina Urrutia A, Huesca-Gómez C, Zamora-González J, Aguilar-Herrera B. Abnormal HDL subclasses distribution in overweight children with insulin resistance or type 2 diabetes mellitus. Clin Chim Acta. 2007 Feb;376(1-2):17-22. doi: 10.1016/j.cca.2006.07.003. Epub 2006 Jul 14. PMID: 16934792.
- Pirillo A, Norata GD, Catapano AL. High-density lipoprotein subfractions--what the clinicians need to know. Cardiology. 2013;124(2):116-25. doi: 10.1159/000346463. Epub 2013 Feb 20. PMID: 23428644.
- Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011 Oct;17(10):594-603. doi: 10.1016/j.molmed.2011.05.013. Epub 2011 Aug 10. PMID: 21839683.
- Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L, Jessup W. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015 Mar 27;116(7):1133-42. doi: 10.1161/CIRCRESAHA.116.305485. Epub 2015 Jan 14. Erratum in: Circ Res. 2015 Jul 17;117(3):e40. PMID: 25589556.
- Davidson WS, Heink A, Sexmith H, Dolan LM, Gordon SM, Otvos JD, Melchior JT, Elder DA, Khoury J, Geh E, Shah AS. Obesity is associated with an altered HDL subspecies profile among adolescents with metabolic disease. J Lipid Res. 2017 Sep;58(9):1916-1923. doi: 10.1194/jlr.M078667. Epub 2017 Jul 25. PMID: 28743729; PMCID: PMC5580903.
- Montero D, Dutheil F, Walther G, Perez-Martin A, Soto-Esclapez L, Vinet A, Roche E. Changes in the profile of circulating HDL subfractions in severe obese adolescents following a weight reduction program. Nutr Metab Cardiovasc Dis. 2021 May 6;31(5):1586-1593. doi: 10.1016/j.numecd.2021.01.025. Epub 2021 Feb 10. PMID: 33810960.
- da Silva SGL, Terreri MT, Abad TTO, Machado D, Fonseca FLA, Hix S, Suano-Souza FI, Sarni ROS, Len CA. The effect of nutritional intervention on the lipid profile and dietary intake of adolescents with juvenile systemic lupus erythematosus: a randomized, controlled trial. Lupus. 2018 Apr;27(5):820-827. doi: 10.1177/0961203317751851. Epub 2018 Jan 10. PMID: 29320971.
- Ryder JR, Vega-López S, Ortega R, Konopken Y, Shaibi GQ. Lifestyle intervention improves lipoprotein particle size and distribution without weight loss in obese Latino adolescents. Pediatr Obes. 2013 Oct;8(5):e59-63. doi: 10.1111/j.2047-6310.2013.00162.x. Epub 2013 Apr 10. PMID: 23576420; PMCID: PMC3898800.
- Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, D'Agostino RB, Wilson PW, Schaefer EJ. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem. 2004 Jul;50(7):1189-200. doi: 10.1373/clinchem.2004.032763. Epub 2004 Apr 23. PMID: 15107310.
- Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am J Epidemiol. 1991 May 1;133(9):884-99. doi: 10.1093/oxfordjournals.aje.a115968. PMID: 2028978.
- El Harchaoui K, Arsenault BJ, Franssen R, Després JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009 Jan 20;150(2):84-93. doi: 10.7326/0003-4819-150-2-200901200-00006. PMID: 19153411.
- St-Pierre AC, Cantin B, Dagenais GR, Mauriège P, Bernard PM, Després JP, Lamarche B. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Québec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005 Mar;25(3):553-9. doi: 10.1161/01.ATV.0000154144.73236.f4. Epub 2004 Dec 23. PMID: 15618542.
- Fisberg RM, Sales CH, Fontanelli MM, Pereira JL, Alves MCGP, Escuder MML, César CLG, Goldbaum M. 2015 Health Survey of São Paulo with Focus in Nutrition: Rationale, Design, and Procedures. Nutrients. 2018 Feb 1;10(2):169. doi: 10.3390/nu10020169. PMID: 29389885; PMCID: PMC5852745.
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253. PMID: 11234459.
- World Health Organization (WHO). Growth reference data for 5-19 years; World Health Organization: Geneva, Switzerland, 2007.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003 Aug;35(8):1381-95. doi: 10.1249/01.MSS.0000078924.61453.FB. PMID: 12900694.
- Matsudo S, Araújo T, Matsudo V, Andrade D, Andrade E, Oliveira LC, Braggion G. International physical activity questionnaire (IPAQ): Validity and reproducibility study in Brazil. Physical Activity Health. 2001;6:5-18.
- World Health Organization (WHO). Global recommendations on physical activity for health; World Health Organization: Geneva, Switzerland, 2010.
- Babor TF, Higgins-Biddle JC. Brief intervention for hazardous and harmful drinking: a manual for use in primary care. Geneva: World Health Organization; 2001.
- Lima CT, Freire AC, Silva AP, Teixeira RM, Farrell M, Prince M. Concurrent and construct validity of the audit in an urban brazilian sample. Alcohol Alcohol. 2005 Nov-Dec;40(6):584-9. doi: 10.1093/alcalc/agh202. Epub 2005 Sep 5. PMID: 16143704.
- Faludi AA, Izar MCO, Saraiva JFK, Chacra APM, Bianco HT, Afiune A Neto, Bertolami A, Pereira AC, Lottenberg AM, et al. Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose – 2017. Arq Bras Cardiol. 2017 Jul;109(2 Supl 1):1-76. Portuguese. doi: 10.5935/abc.20170121. Erratum in: Arq Bras Cardiol. 2017 Nov;109 (5):499. PMID: 28813069.
- Sanches LB, da Silva IT, Paz AF, Fisberg M, Cintra IP, Villar BS, Damasceno NR. Anti-oxLDL autoantibodies and their correlation with lipid profile and nutritional status in adolescents. J Pediatr (Rio J). 2008 May-Jun;84(3):258-63. doi: 10.2223/JPED.1805. PMID: 18535732.
- Bauman CD, Bauman JM, Mourão DM, Pinho L, Brito MFSF, Carneiro ALG, Silveira MF, Silva CSOE. Dyslipidemia prevalence in adolescents in public schools. Rev Bras Enferm. 2020 Apr 22;73(3):e20180523. English, Portuguese. doi: 10.1590/0034-7167-2018-0523. PMID: 32321121.
- Tomeleri CM, Ronque ER, Silva DR, Cardoso Júnior CG, Fernandes RA, Teixeira DC, Barbosa DS, Venturini D, Okino AM, Oliveira JA, Cyrino ES. Prevalence of dyslipidemia in adolescents: comparison between definitions. Rev Port Cardiol. 2015 Feb;34(2):103-9. English, Portuguese. doi: 10.1016/j.repc.2014.08.020. Epub 2015 Feb 3. PMID: 25660460.
- Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019 Dec;70(6). doi: 10.26402/jpp.2019.6.01. Epub 2020 Feb 19. PMID: 32084643.
- Rizzo M, Berneis K, Zeljkovic A, Vekic J. Should we routinely measure low-density and high-density lipoprotein subclasses? Clin Lab. 2009;55(11-12):421-9. PMID: 20225664.
- Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism. 2006 Jan;55(1):113-8. doi: 10.1016/j.metabol.2005.07.016. PMID: 16324929.
- Sessa WC. Estrogen Reduces LDL (Low-Density Lipoprotein) Transcytosis. Arterioscler Thromb Vasc Biol. 2018 Oct;38(10):2276-2277. doi: 10.1161/ATVBAHA.118.311620. PMID: 30354224; PMCID: PMC6448576.
- Choi R, Lee SG, Lee EH. Lipoprotein(a) in the Korean Pediatric Population Visiting Local Clinics and Hospitals. Nutrients. 2022 Jul 8;14(14):2820. doi: 10.3390/nu14142820. PMID: 35889777; PMCID: PMC9320048.
- Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engström G, Williams PT, Kathiresan S, Melander O, Krauss RM. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009 Nov;29(11):1975-80. doi: 10.1161/ATVBAHA.109.190405. Epub 2009 Sep 3. PMID: 19729614; PMCID: PMC2772123.
- Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM. Systematic review: association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med. 2009 Apr 7;150(7):474-84. doi: 10.7326/0003-4819-150-7-200904070-00007. PMID: 19349632; PMCID: PMC6880859.
- Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem. 1992 Sep;38(9):1632-8. PMID: 1326420.
- Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008 Aug;54(8):1307-16. doi: 10.1373/clinchem.2007.100586. Epub 2008 May 29. PMID: 18515257.
- Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982 Jan;23(1):97-104. PMID: 7057116.
- Williams PT, Zhao XQ, Marcovina SM, Otvos JD, Brown BG, Krauss RM. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis. 2014 Apr;233(2):713-720. doi: 10.1016/j.atherosclerosis.2014.01.034. Epub 2014 Jan 30. PMID: 24603218; PMCID: PMC3990359.
- Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009 Feb 24;119(7):931-9. doi: 10.1161/CIRCULATIONAHA.108.816181. Epub 2009 Feb 9. PMID: 19204302; PMCID: PMC2663974.
- Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol. 2015 Oct 5;6:218. doi: 10.3389/fphar.2015.00218. PMID: 26500551; PMCID: PMC4593254.
- Julve J, Llaverias G, Blanco-Vaca F, Escolà-Gil JC. Seeking novel targets for improving in vivo macrophage-specific reverse cholesterol transport: translating basic science into new therapies for the prevention and treatment of atherosclerosis. Curr Vasc Pharmacol. 2011 Mar;9(2):220-37. doi: 10.2174/157016111794519264. PMID: 21143175.
- Christensen JJ, Ulven SM, Retterstøl K, Narverud I, Bogsrud MP, Henriksen T, Bollerslev J, Halvorsen B, Aukrust P, Holven KB. Comprehensive lipid and metabolite profiling of children with and without familial hypercholesterolemia: A cross-sectional study. Atherosclerosis. 2017 Nov;266:48-57. doi: 10.1016/j.atherosclerosis.2017.09.021. Epub 2017 Sep 21. PMID: 28963918.
- Davidson WS, Heink A, Sexmith H, Dolan LM, Gordon SM, Otvos JD, Melchior JT, Elder DA, Khoury J, Geh E, Shah AS. Obesity is associated with an altered HDL subspecies profile among adolescents with metabolic disease. J Lipid Res. 2017 Sep;58(9):1916-1923. doi: 10.1194/jlr.M078667. Epub 2017 Jul 25. PMID: 28743729; PMCID: PMC5580903.
- Gordon SM, Davidson WS, Urbina EM, Dolan LM, Heink A, Zang H, Lu LJ, Shah AS. The effects of type 2 diabetes on lipoprotein composition and arterial stiffness in male youth. Diabetes. 2013 Aug;62(8):2958-67. doi: 10.2337/db12-1753. Epub 2013 Jul 8. Erratum in: Diabetes. 2016 Jul;65(7):2100. PMID: 23835332; PMCID: PMC3717874.
- Muchová J, Andrezálová L, Oravec S, Nagyová Z, Garaiova I, Ďuračková Z. High density lipoprotein subfractions and paraoxonase 1 in children. Acta Biochim Pol. 2016;63(3):555-63. doi: 10.18388/abp.2015_1230. Epub 2016 Jun 6. PMID: 27262841.
- Burlutskaya AV, Tril VE, Polischuk LV, Pokrovskii VM. Dyslipidemia in pediatrician's practice. Rev Cardiovasc Med. 2021 Sep 24;22(3):817-834. doi: 10.31083/j.rcm2203088. PMID: 34565080.
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011 Dec;128 Suppl 5(Suppl 5):S213-56. doi: 10.1542/peds.2009-2107C. Epub 2011 Nov 14. PMID: 22084329; PMCID: PMC4536582.
- Brazilian Society of Cardiology. Brazilian Cardiology Archives. 2017;109(1).
- Fryar CD, Kruszon-Moran D, Gu Q, Carroll M, Ogden CL. Mean Body Weight, Height, Waist Circumference, and Body Mass Index Among Children and Adolescents: United States, 1999-2018. Natl Health Stat Report. 2021 Aug;(160):1-24. PMID: 34520341.
- Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018 Aug 31;17(1):122. doi: 10.1186/s12933-018-0762-4. PMID: 30170598; PMCID: PMC6119242.