More Information
Submitted: April 18, 2025 | Approved: April 28, 2025 | Published: April 29, 2025
How to cite this article: Mezdaoui I, Isfaoun Z, El-Hafidi N, Tligui H, Kili A, Hessissen L. Invasive Magnusiomyces Capitatus Infection in a Patient Followed for Acute Myeloblastic Leukemia: A Case Report. J Adv Pediatr Child Health. 2025; 8(1): 010-014. Available from:
https://dx.doi.org/10.29328/journal.japch.1001072
DOI: 10.29328/journal.japch.1001072
Copyright License: © 2025 Mezdaoui I, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Invasive fungal infections; Acute myeloid leukemia; Magnusiomyces capitatus; Case report
Invasive Magnusiomyces Capitatus Infection in a Patient Followed for Acute Myeloblastic Leukemia: A Case Report
Imane Mezdaoui1*, Zineb Isfaoun2, Naima El-Hafidi3, Houssain Tligui4, Amina Kili2 and Laila Hessissen2
1General Pediatrics, Rabat Children Hospital, Morocco
2Pediatric Oncology, Rabat Children Hospital, Morocco
3Pediatric Pulmonology, Immunoallergology and Infectious Diseases, Rabat Children Hospital, Morocco
4Research Laboratory, Rabat Children Hospital, Morocco
*Address for Correspondence: Imane Mezdaoui, MD, General Pediatrics, Rabat Children Hospital, Morocco, Email: [email protected]
Magnusiomyces capitatus is a rare cause of invasive fungal infection in immuno-compromised patients. We report the case of magnusiomyces infection of the central nervous system, the lungs and sinus with a palatal lesion, in a patient treated for acute myeloid leukemia. While Magnusiomyces infections pose diagnostic and therapeutic challenges, a comprehensive understanding of their epidemiology, clinical manifestations, and microbiological aspects is essential to guide effective management. The patient improved under antifungal treatments despite a reduced sensitivity of the different antifungals to the antifungogram. The important delays between chemotherapy cycles and its reduced intensity due to the Magnusiomyces infection has made managing the anticancer treatment more challenging.
The challenges in treating pediatric Acute Myeloid Leukemia (AML) are clearly apparent, especially in Low- and Middle-Income Countries (LMICs), where mortality rates associated with treatment can reach alarming levels due to both disease progression and treatment toxicity. Despite AML accounting for a significant portion of childhood leukemias worldwide, survival disparities persist between resource-rich and resource-poor countries [1].
One significant factor contributing to mortality rates in pediatric acute myeloid leukemia (AML) is the heightened susceptibility to infections. These infections can range from bacterial and viral to fungal, posing a serious threat to the already compromised survival [1].
Infections can occur as a result of treatment-induced neutropenia, where the body’s ability to fight off pathogens is significantly diminished due to low levels of neutrophils, a type of white blood cell essential for combating infections. Furthermore, prolonged hospital stays and invasive procedures, such as central venous catheter insertion, further increase the risk of acquiring infections.
Among the infectious agents, Infections caused by Magnusiomyces capitatus, formerly known as Geotrichum capitatum, are rare but can be serious, particularly in immunocompromised individuals [2]. This underscores the importance of reporting new cases of this infection. In this context, we present a case of M. capitatus infection with triple localization in the brain, sinuses, and lungs in a child with acute myeloid leukemia. Typically, M. capitatus infection manifests as septicemia with occasional secondary sites [3]. Despite adequate antifungal therapy, this disease has been associated with a 50% mortality rate [2,3].
A 10-year-old boy presented with acute pain in the left hypochondrium, abdominal distension, vomiting, and respiratory discomfort, along with fatigue, weight loss, and unspecified fever, fifteen days prior to admission. Upon admission, the patient exhibited cachexia, stunted growth with weight at -3 Standard Deviations (SD) and height at -2 SD, purpuric spots, hepatosplenomegaly, bilateral retro-auricular and inguinal lymphadenopathy, enlarged tonsils, and gums.
Initial investigations revealed a hemoglobin level of 9 g/dl, white blood cell count of 22300 elements/mm³, neutrophil count at 7100 element/mm³, lymphocytes at 10200 elements/mm³, monocytes at 4500 elements/mm³, and thrombocytopenia at 22000 elements/mm³. A bone marrow examination showed 35% blasts, with immunophenotyping indicating acute myeloid leukemia with aberrant CD7 expression at 66%. Cytogenetic analysis revealed a normal karyotype. The chest X-ray and abdominal ultrasound were normal except for hepatosplenomegaly. Cerebral fluid study was normal.
The patient was diagnosed with Acute Myeloid Leukemia (AML) and underwent induction therapy according to the national adapted treatment regimen the “AML-MA 2011” protocol with 5 treatments 28 days’ time interval.
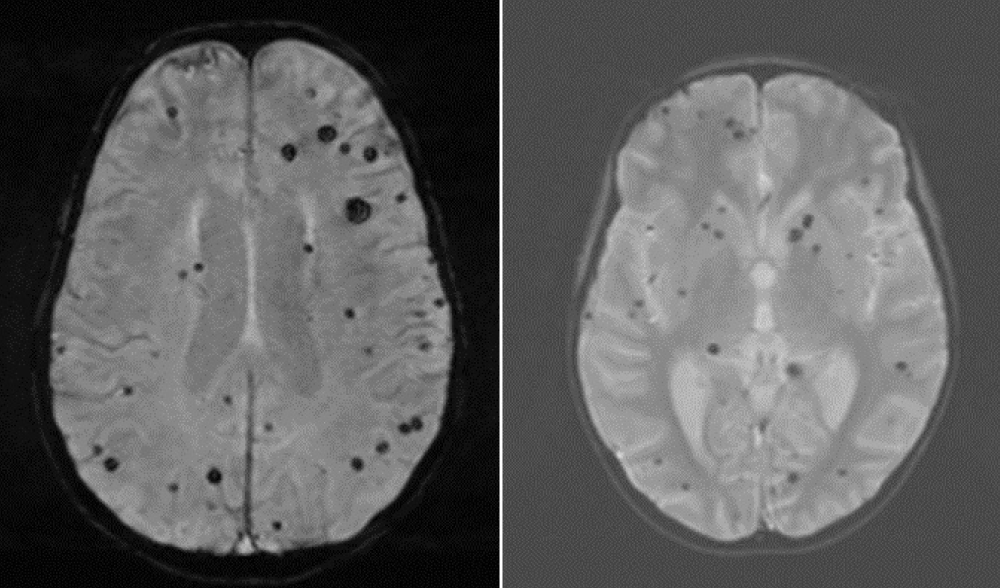
On day 7 of the first induction, the patient developed intense headaches with photophobia, but a brain scan showed no abnormalities. On day14, developed a neutropenic fever, and received ceftazidime. On day 15, a generalized tonic-clonic seizure occurred, managed with midazolam and subsequent antiepileptic therapy. Brain CT remained normal. An MRI two days after the seizure confirmed multiple signal abnormalities of the white matter sub and juxta cortical, supra and sub tentorial, and U-shaped, bi-hemispherical fibers, asymmetric in hyperT2 and Flair, some of which restrict diffusion with low ADC, not enhancing after injection of Gado. It is associated with an asymmetrical demyelinating attack of the central ganglia, right lateropontine, bulbar and cervical medullary lateralized to the left, presenting the same semiological characteristics (Figure 1).
Figure 1: Brain MRI showing bilateral and asymmetrical demyelinating lesions above and below the tentorial and cervical spinal cord.
Voriconazole was initiated on day 22. Because of persistent febrile neutropenia with neutrophils at 0 on day 23, ceftazidime was stopped, and imipenem and vancomycin were started. On day 28, a control brain CT revealed regression in size and number of small oval lesions and certain punctiform spontaneously hyperdense intraparenchymal bilateral lesions more marked on the left the largest is left parietal.
On day 31, the patient became afebrile, and antibiotics were stopped. A second brain CT on day 37 showed a marked regression of parenchymal lesions. A second induction was initiated on day 44. A control brain MRI revealed the persistence of multiple lesions with asymmetrical distribution.
On day 61, the patient developed fever and neutropenia, leading to antibiotic therapy adjustments with imipenem and vancomycin. On day 71, mucositis was observed, prompting the addition of fluconazole. On day 74, facial MRI revealed ethmoido-spheno-maxillary sinusitis.
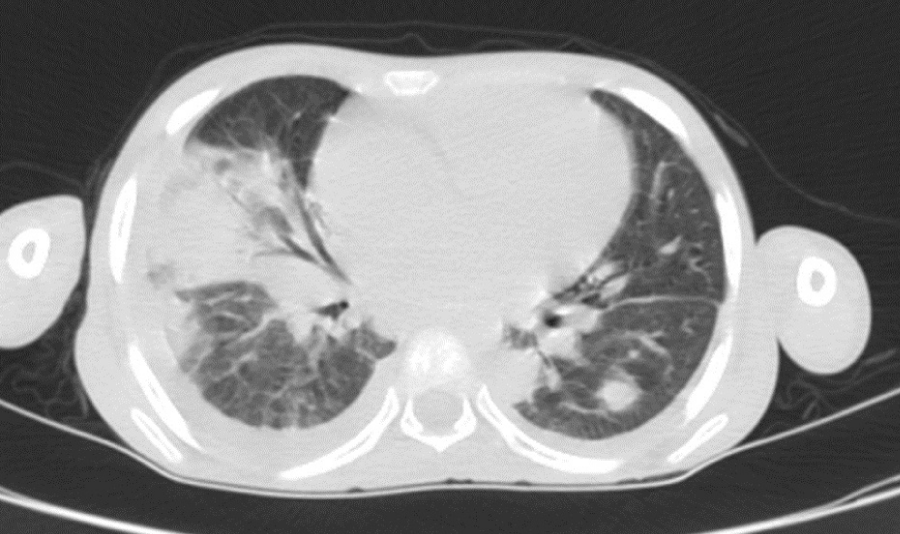
On day 75, abdominal distension with abundant ascites requiring drainage and respiratory discomfort led to a thoraco-abdomino-pelvic CT, condensation in the two pulmonary fields, containing hypodense formations of fluid density surrounded by enhanced shells after injection of contrast agent and bilateral pleural effusion of low abundance in favor of multiple bilateral lung abscesses and bilateral pleurisy of low abundance (Figure 2) with large peritoneal effusion with thickening of the peritoneal layers and a bronchoalveolar lavage detected Klebsiella pneumonia, the treatment was continued.
Figure 2: Pulmonary CT shows Multiple bilateral lung abscesses with bilateral pleurisy of low abundance and peritoneal effusion of large abundance with thickening of the peritoneal layers.
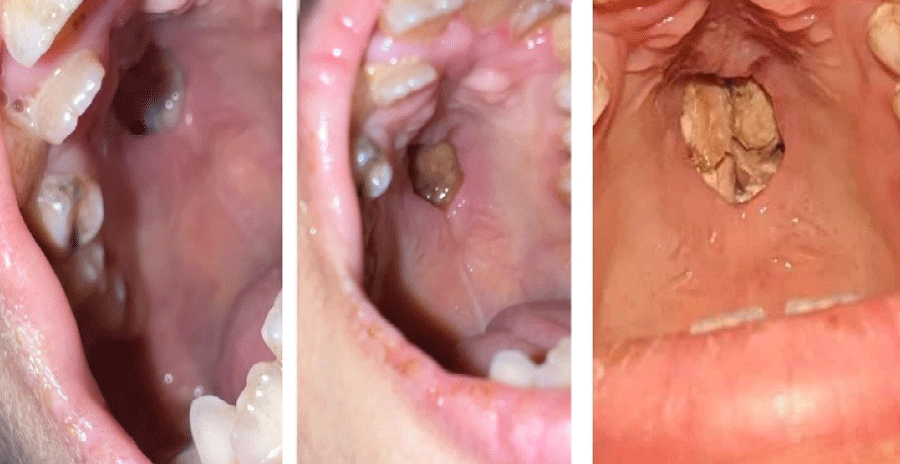
On day 82, vancomycin was discontinued, and three days after, the patient remained afebrile, allowing the cessation of imipenem/cilastatin. A second brain MRI on day 89 suggested stable lesions. On day 95, a marrow examination revealed 1% blasts. On day 97, the patient developed a midline palatal fistula (Figure 3).
Figure 3: Loss of oval substance at the level of the palate and communication with the nasal cavities.
On day 101, mycological tests of the bronchoalveolar lavage confirmed Magnusiomyces Capitatus resistant to amphotericin B, intermediate sensitivity to fluconazole and the sensibility to voriconazole has not been studied in the antifungogram because the reagent is not available. A deep infection with Magnusiomyces Capitatus involving the brain, sinuses, and lungs was diagnosed and we decided to start intraveinous voriconazole for fifteen days.
Then the patient has been on oral voriconazole for four months, and we were unable to continue with consolidations as per the established protocol, especially due to the presence of the midline palatal fistula for which maxillofacial surgeons placed a prosthesis. The patient continued chemotherapy in the form of five days of low-dose aracytin (100 mg/m2/day) per month and continuous purinethol for 4 cycles. There was a clear improvement in infectious lesions in the lungs and sinuses with persistence of brain lesions, and he remained in complete remission of his leukemia after 4 cycles of aracytin and purinethol.
We present the case of a 10-year-old boy undergoing treatment for acute myeloblastic leukemia, who developed an invasive Magnusiomyces capitatus infection. This case posed significant challenges in both diagnosis and treatment, exacerbated by the limited resources and conditions characteristic of a Low-Income Country (LIC). The primary challenge in managing this patient was establishing a definitive diagnosis. Despite non-specific brain MRI findings suggestive of Magnusiomyces capitatus infection, standard diagnostic procedures such as blood culture and mycological examination of sputum were inconclusive. Ultimately, the diagnosis was confirmed through mycological analysis of bronchoalveolar lavage fluid using specialized media. Another obstacle was the difficulty in accessing voriconazole and ensuring therapeutic compliance. Additionally, managing concurrent chemotherapy and infection presented a complex clinical scenario, compounded by the patient’s challenging oncological prognosis. Moreover, addressing the aftermath of dental surgery to repair palate defects further complicated the patient’s care. Geotrichum fungi are cosmopolitan, ubiquitous saprophytes found in nature, particularly on plants, fruits, vegetables, and some animal products like dairy [1]. M. capitatus is frequently isolated from the oral cavity and stools in healthy individuals, mainly presenting as septicemia with occasional localizations [3,4]. It can be inhaled, causing pulmonary and alveolar pathogenicity, as well as inducing multivisceral lesions [1]. In neutropenic patients, M. capitatus causes infections similar to candidiasis and other invasive fungal infections [5]. Overall, 60% to 80% of patients develop deep organ involvement, affecting the lungs, kidneys, liver, spleen, brain, and endocardium [6-8]. Despite appropriate antifungal therapy, it has been associated with a mortality rate exceeding 50%, reaching 75% in oncology patients and 40% in other groups [5]. Magnusiomyces is a genus of arthroconidial yeasts belonging to the order Saccharomycetales, family Dipodascaceae, and is phylogenetically distant from ascomycetous yeasts producing budding cells. The species in this genus were previously known as Geotrichum/Dipodascus (for the anamorph and teleomorph, respectively) [2]. However, with the abandonment of dual nomenclature for fungi, the name Magnusiomyces was retained for filamentous yeasts producing bipodal asci containing ascospores wrapped in gelatinous sheaths [3].
Currently, the genus comprises 14 species. The clinically significant species is Magnusiomyces capitatus. Patients with hematological malignancies or Hematopoietic Stem Cell Transplantation (HSCT) are at higher risk, especially in the presence of predisposing factors [5]. While Candida and Aspergillus are the most common pathogens in these patients, lesser-known fungi like Magnusiomyces are gaining importance due to increasing incidence and limited treatment options. In hematology-oncology services, Magnusiomyces yeasts represent 5% of non-Candida and Cryptococcus fungi [5]. According to MEDLINE research from 1965 to 2011, there were 202 reports on Geotrichum, Blastoschizomyces, and Trichosporon capitatum, including 186 cases of invasive infections with a mortality rate of up to 50%. The youngest patient reported was only 7 years old [6]. M. capitatus, the most isolated species, is mainly described in Europe, particularly in the Mediterranean region, with 87% of cases, suggesting the influence of climatic factors in the emergence of these infections [5,7,8].
Magnusiomyces IFIs are relatively rare, with around 100 cases reported in patients with hematological malignancies [5]. Entry points can be through the digestive, pulmonary, or cutaneous routes as is the case of our patient [7]. Clinical manifestations of M. capitatus infection are similar to other fungal infections. Most patients develop agranulocytosis and fever, showing poor response to broad-spectrum antibiotic treatments as is the case of our patient [6]. M. capitatus infection is mainly a disseminated systemic infection, but in non-agranulocytic patients, it often presents as focal infections like endocarditis, meningitis, osteomyelitis, or intervertebral disc infection [6]. Distinguishing M. capitatus infection clinically from Candida and Aspergillus infections is challenging [6]. Deep organ involvement occurs in 60% to 80% of M. capitatus infected patients [1].
Imaging results are non-specific, but imaging studies are moderately useful in assessing the extent of the disease, especially in the lungs, eyes, skin, heart, and skeleton. Follow-up imaging is recommended to monitor the therapeutic response and not to make the diagnosis [5] and that was the whole difficulty with our patient. M. capitatus is frequently isolated from the respiratory tract, and pulmonary colonization can lead to infection [4,6]. Diagnosis involves detecting the fungus in sputum or bronchial aspirate in patients with pneumonia without sepsis [1,2]. However, isolated pulmonary forms seem rare [1].
In patients with M. capitatus septicemia, the lungs are often involved, with typical radiographic features like halo signs and crescent signs [6]. High-resolution lung CT can reveal inflammatory infiltrates, pleural effusion, nodules, alveolar consolidation with air bronchograms, and subsegmental atelectasis [5,7]. When this organism is abundantly isolated exclusively with a positive direct examination and suggestive clinical symptoms, its involvement as a pulmonary pathogen is considered [1,2].
Reports suggest that M. capitatus can cause brain lesions, but specific types are not well-described [5,6]. Brain CT in a reported case revealed multiple hypodensity zones with postmortem mycological diagnosis of M. capitatus in a 19-year-old woman with myelodysplastic syndrome [7]. For ocular or cerebral infections, MRI is moderately recommended to define the disease [1-5]. Skin and mucosal involvement in disseminated M. capitatus infection is common, and the skin can serve as an entry point [1-3]. Lesions initially present as purpuric nodules that can progress to central necrotic lesions, potentially involving oral and pharyngeal mucosa [6]. The fungus can be identified through skin biopsy and/or culture, although skin biopsy is often sterile [1-4]. Ultrasound can reveal multiple hypoechoic nodules in the liver, spleen and kidneys in M. capitatus infections [3,4].
In our patient, abdominal ultrasound and CT did not detect nodules but significant hepatomegaly. Several cases of peritonitis have been reported in immunocompetent and immunocompromised subjects, often diagnosed postoperatively [7,8]. Our patient presented with abundant ascites requiring drainage but no germs were isolated from the ascitic fluid. Also note that several cases of Magnusiomyces capitatus endocarditis have been reported. Transesophageal echocardiography is recommended to confirm endocarditis [1-5]. Diagnosis is typically established through blood culture, positive in over 70% of patients [6]. In our case, no blood culture was positive which made the microbiological diagnosis more difficult. M. capitatus antigens may cross-react in Aspergillus galactomannan assays, and galactomannan can be detected in some patients [1-4].
To date, there is no established antifungal treatment for invasive Magnusiomyces infections, mainly due to the infrequency of this species, challenging diagnosis by conventional methods, and lack of antifungal thresholds [1]. Treatment should be initiated promptly. While there is insufficient clinical data to assess the optimal treatment for M. capitatus in hematological patients, based on in vitro sensitivity and limited clinical data, any formulation of amphotericin B, with or without flucytosine, may be recommended [7]. Voriconazole and 5-fluorocytosine can also be used, either as monotherapy or in combination [2,7]. M. capitatus is considered intrinsically resistant to echinocandins, such as caspofungin [7].
Currently, the best outcomes in treatment seem to be achieved with combinations of antifungals, like amphotericin B combined with 5-fluorocytosine or itraconazole [1]. The combination of liposomal amphotericin B and itraconazole has been successfully used in a case of disseminated M. capitatus infection, with the liposomal form reducing the adverse effects of amphotericin B [1]. In patients, overall health status, particularly rapid recovery from neutropenia, can significantly influence the effectiveness of treatment, irrespective of the inherent efficacy of antifungal therapies [8]. In our case, M. capitatus exhibited resistance to amphotericin B, prompting the administration of voriconazole, which resulted in notable improvement after three months of oral treatment. However, voriconazole is not readily accessible, posing challenges in medication procurement. M. capitatus is considered intrinsically resistant to echinocandins, such as caspofungin [7]. Currently, the best outcomes in treatment seem to be achieved with combinations of antifungals, like amphotericin B combined with 5-fluorocytosine or itraconazole [1]. The combination of liposomal amphotericin B and itraconazole has been successfully used in a case of disseminated M. capitatus infection, with the liposomal form reducing the adverse effects of amphotericin B [1]. In patients, overall health status, particularly rapid recovery from neutropenia, can significantly influence the effectiveness of treatment, irrespective of the inherent efficacy of antifungal therapies [8].
In our case, M. capitatus exhibited resistance to amphotericin B, prompting the administration of voriconazole, which resulted in notable improvement after three months of oral treatment. However, voriconazole is not readily accessible, posing challenges in medication procurement. In low-income countries, antifungal accessibility remains a critical barrier, as newer antifungals like voriconazole, posaconazole, and liposomal amphotericin B are often either unavailable or prohibitively expensive, limiting treatment options to less effective or more toxic alternatives. Additionally, susceptibility testing for rare fungi such as M. capitatus is not routinely available, complicating the ability to tailor antifungal therapy based on resistance profiles. These limitations contribute to delayed treatment initiation and increased mortality. In our patient’s case, the difficulties in sourcing voriconazole necessitated exceptional efforts, reflecting broader systemic issues faced in resource-limited settings.
Additionally, deviation from the standard chemotherapy protocol occurred, with the patient missing Consolidation. Instead, the patient received five days of aracytin per month along with continuous purinethol, resulting in favorable progress both in managing the infection and controlling leukemia. Nevertheless, uncertainties persist regarding the overall prognosis, particularly due to the deviation from the chemotherapy regimen. Additionally, deviation from the standard chemotherapy protocol occurred, with the patient missing consolidation. Instead, the patient received five days of aracytin per month along with continuous purinethol, resulting in favorable progress both in managing the infection and controlling leukemia. Nevertheless, uncertainties persist regarding the overall prognosis, particularly due to the deviation from the chemotherapy regimen.
This report underscores the clinical significance of invasive infections caused by Magnusiomyces capitatus, highlighting the diagnostic challenges, diverse clinical presentations, and treatment complexities associated with these infections. The rarity of Magnusiomyces infections, coupled with diagnostic hurdles and the absence of established antifungal guidelines, adds to the complexity of managing these cases.
Despite these obstacles, prompt initiation of treatment is crucial. Moreover, the impact of Magnusiomyces infections extends beyond antifungal efficacy, with overall patient health, particularly rapid recovery from neutropenia, significantly influencing treatment outcomes. The rising incidence of invasive fungal infections, especially in patients with hematological malignancies or undergoing stem cell transplantation, underscores the need for heightened vigilance and continued research efforts to enhance infection management. In summary, a comprehensive understanding of Magnusiomyces infections, including their epidemiology, clinical manifestations, and microbiological aspects, is essential for effective management. Ongoing research endeavors are necessary to develop precise diagnostic strategies and more effective treatments, ultimately improving the prognosis for patients with these rare yet potentially serious infections.
Scientific responsibility statement
Authors declare that they are responsible for the article’s scientific content. These responsibility areas include study design, data collection, analysis and interpretation, writing, preparation and scientific review of the contents, and approval of the final version of the article.
Authors state that the submitted article, either in full or in part, has not been previously published or is not being assessed for publication in either printed form or as digital media.
Patient anonymity and informed consent statement
Informed written consent was obtained from the patient’s legal guardian for publication of this case report, including clinical details and accompanying images. All efforts have been made to ensure patient anonymity, and no identifying information has been included in the manuscript. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Ethical statement
Ethical approval was not required for this case report, as per the policies regarding single-patient case reports. Written informed consent for publication was obtained from the patient’s legal guardian.
Ethical approval and consent to participate
Ethical approval was not required for this single case report in accordance with the policies. Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images.
- Bansal D, Davidson A, Supriyadi E, Njuguna F, Ribeiro RC, Kaspers GJL. SIOP PODC adapted risk stratification and treatment guidelines: recommendations for acute myeloid leukemia in resource-limited settings. Pediatr Blood Cancer. 2023;70(11):e30410. Available from: https://doi.org/10.1002/pbc.28087
- Zhu M, Yan L, de Hoog S, Liao W, Zhang H, Zhao R. Invasive infections due to Magnusiomyces capitatus: case report and review of its prevalence in China. Mycology. 2022;13(1):76–80. Available from: https://doi.org/10.1080/21501203.2021.2000059
- Kaplan E, Al-Hatmi AM, Ilkit M, Gerrits van den Ende A, Hagen F, Meis JF. Molecular diagnostics of arthroconidial yeasts, frequent pulmonary opportunists. J Clin Microbiol. 2018;56(1):e01144-17. Available from: https://doi.org/10.1128/jcm.01427-17
- de Hoog S, Smith M. Ribosomal gene phylogeny and species delimitation in Geotrichum and its teleomorphs. Stud Mycol. 2004;50:489–515. Available from: https://www.studiesinmycology.org/sim/Sim50/046-Ribosomal_gene_phylogeny_and_species_delimitation_in_Geotrichum_and_its_teleomorphs.pdf
- Rouis S, Khammeri I, Achour B, Achour A, Ben Sayed N, Regaieg H, et al. Invasive infection caused by Geotrichum capitatum in three patients with acute myeloid leukemia. Pan Afr Med J Clin Med. 2020;4:1–5. Available from: https://www.clinical-medicine.panafrican-med-journal.com/content/article/4/41/full/
- Gao GX, Tang HL, Zhang X, Xin XL, Feng J, Chen XQ. Invasive fungal infection caused by Geotrichum capitatum in patients with acute lymphoblastic leukemia: a case study and literature review. Int J Clin Exp Med. 2015;8(8):14228–35. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4613086/
- Tanuskova D, Horakova J, Svec P, Bodova I, Lengerova M, Bezdicek M. First case of invasive Magnusiomyces capitatus infection in Slovakia. Med Mycol Case Rep. 2017;16:12–15. Available from: https://doi.org/10.1016/j.mmcr.2017.03.004
- Ortiz-Álvarez J, Reséndiz-Sánchez J, Juárez-Montiel M, Hernández-García JA, Vázquez-Guerrero E, Hernández-Rodríguez C, et al. Invasive fungal infection caused by Magnusiomyces capitatus in an immunocompromised pediatric patient with acute lymphoblastic leukemia in Mexico City: a case report. J Fungi (Basel). 2022;8(8):851. Available from: https://doi.org/10.3390/jof8080851